Abstract
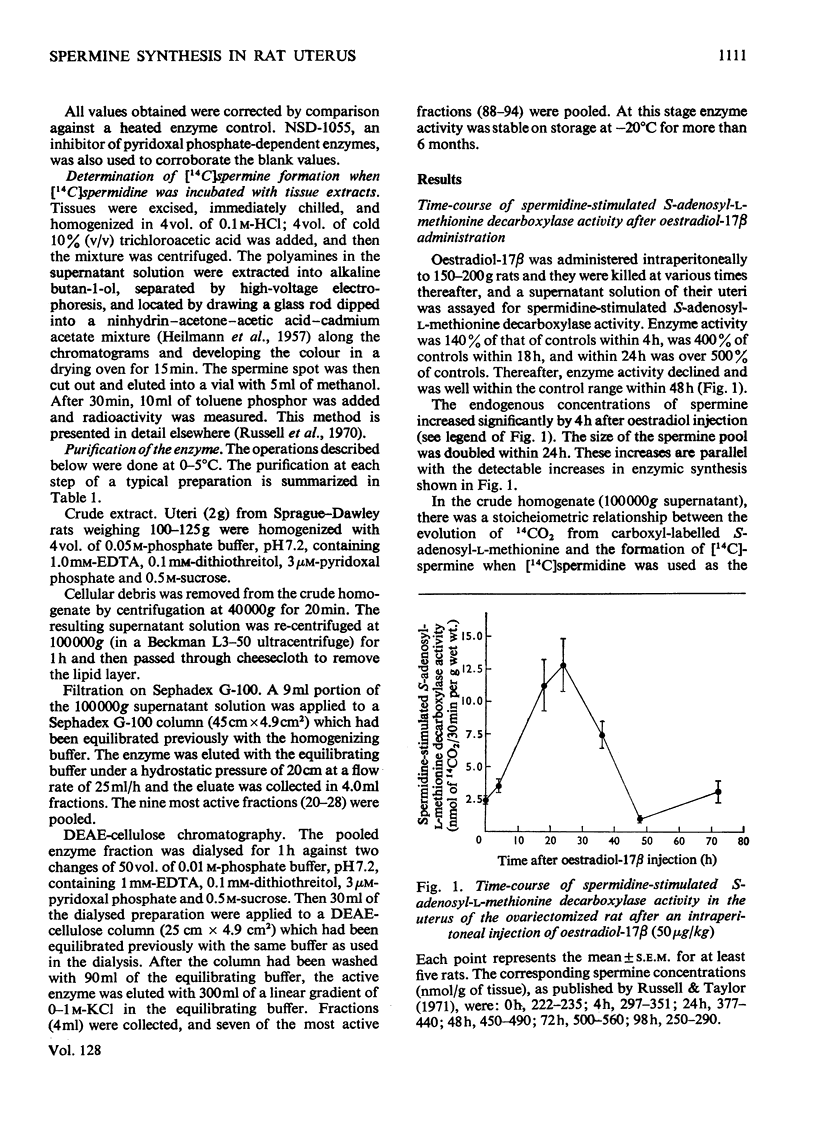
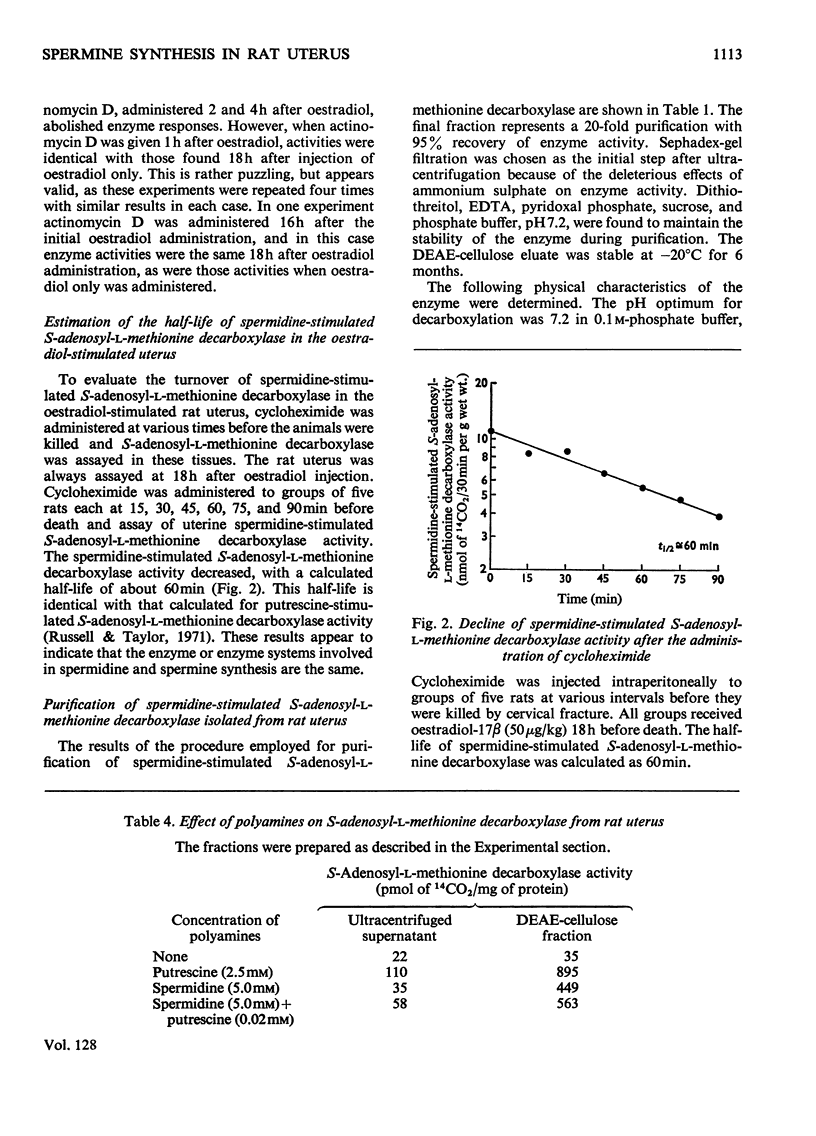
We reported that spermidine and spermine pools in the uterus both doubled within 24h after oestradiol administration to castrated rats (Russell & Taylor, 1971). Now we have studied the enzymic synthesis of spermine (by spermidine-dependent S-adenosyl-l-methionine decarboxylase) and find that the activity of the enzyme(s) involved is elevated soon after hormone administration. Enzyme activity is increased within 4h and is five times that of controls within 24h. Cycloheximide or actinomycin D administered at the time of oestradiol injection completely blocked the increase in enzyme activity. The enzyme involved in spermine synthesis, S-adenosyl-l-methionine decarboxylase, with S-adenosyl-l-methionine and spermidine as required substrates, was partially purified on Sephadex and DEAE-cellulose columns. The decarboxylation of S-adenosyl-l-methionine could not be separated from the transfer of a propylamine moiety from the decarboxylated S-adenosyl-l-methionine to spermidine to form spermine. We were unable also to separate this system from the enzyme that formed spermidine when S-adenosyl-l-methionine and putrescine are used as substrates. Spermidine-stimulated S-adenosyl-l-methionine decarboxylase has an apparent half-life of 60min, identical with the half-life reported for putrescine-stimulated S-adenosyl-l-methionine decarboxylase. These results strongly suggest that the same enzyme(s) operate in the synthesis of both spermidine and spermine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feldman M. J., Levy C. C., Russell D. H. Purification and characterization of S-adenosyl-L-methionine decarboxylase from rat liver. Biochemistry. 1972 Feb 29;11(5):671–677. doi: 10.1021/bi00755a002. [DOI] [PubMed] [Google Scholar]
- Feldman M. J., Levy C. C., Russell D. H. The purification of S-adenoxyl-L-methionine decarboxylase from rat liver: inability to separate decarboxylation from spermidine synthesis. Biochem Biophys Res Commun. 1971 Aug 6;44(3):675–681. doi: 10.1016/s0006-291x(71)80136-5. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L., STELLA E. J., KNOX W. E. The effect of hydrocortisone on tyrosine-alpha-ketoglutarate transaminase and tryptophan pyrrolase activities in the isolated, perfused rat liver. J Biol Chem. 1962 May;237:1723–1726. [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Janne J., Williams-Ashman H. G., Schenone A. Spermidine synthesizing enzymes in baker's yeast. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1362–1368. doi: 10.1016/s0006-291x(71)80024-4. [DOI] [PubMed] [Google Scholar]
- Jänne J., Raina A. Stimulation of spermidine synthesis in the regenerating rat liver: relation to increased ornithine decarboxylase activity. Acta Chem Scand. 1968;22(4):1349–1351. doi: 10.3891/acta.chem.scand.22-1349. [DOI] [PubMed] [Google Scholar]
- Jänne J., Schenone A., Williams-Ashman H. G. Separation of two proteins required for synthesis of spermidine from S-adenosyl-L-methionine and putrescine in rat prostate. Biochem Biophys Res Commun. 1971 Feb 19;42(4):758–764. doi: 10.1016/0006-291x(71)90552-3. [DOI] [PubMed] [Google Scholar]
- Jänne J. Studies on the biosynthetic pathway of polyamines in rat liver. Acta Physiol Scand Suppl. 1967;300:1–71. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- KENNEY F. T. Induction of tyrosine-alpha-ketoglutarate transaminase in rat liver. IV. Evidence for an increase in the rate of enzyme synthesis. J Biol Chem. 1962 Nov;237:3495–3498. [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Raina A., Hannonen P. Biosynthesis of spermidine and spermine in regenerating rat liver: some properties of the enzyme systems involved. Acta Chem Scand. 1970;24(8):3061–3064. doi: 10.3891/acta.chem.scand.24-3061. [DOI] [PubMed] [Google Scholar]
- Raina A., Jänne J., Siimes M. Stimulation of polyamine synthesis in relation to nucleic acids in regenerating rat liver. Biochim Biophys Acta. 1966 Jul 20;123(1):197–201. doi: 10.1016/0005-2787(66)90173-0. [DOI] [PubMed] [Google Scholar]
- Raina Aarne, Hannonen Pekka. Separation of enzyme activities catalysing spermidine and spermine synthesis in rat brain. FEBS Lett. 1971 Jul 15;16(1):1–4. doi: 10.1016/0014-5793(71)80669-5. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Medina V. J., Snyder S. H. The dynamics of synthesis and degradation of polyamines in normal and regenerating rat liver and brain. J Biol Chem. 1970 Dec 25;245(24):6732–6738. [PubMed] [Google Scholar]
- Russell D. H., Taylor R. L. Polyamine synthesis and accumulation in the castrated rat uterus after estradiol-17-beta stimulation. Endocrinology. 1971 Jun;88(6):1397–1403. doi: 10.1210/endo-88-6-1397. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- SCHIMKE R. T. THE IMPORTANCE OF BOTH SYNTHESIS AND DEGRADATION IN THE CONTROL OF ARGINASE LEVELS IN RAT LIVER. J Biol Chem. 1964 Nov;239:3808–3817. [PubMed] [Google Scholar]
- Schimke R. T., Ganschow R., Doyle D., Arias I. M. Regulation of protein turnover in mammalian tissues. Fed Proc. 1968 Sep-Oct;27(5):1223–1230. [PubMed] [Google Scholar]


