Abstract
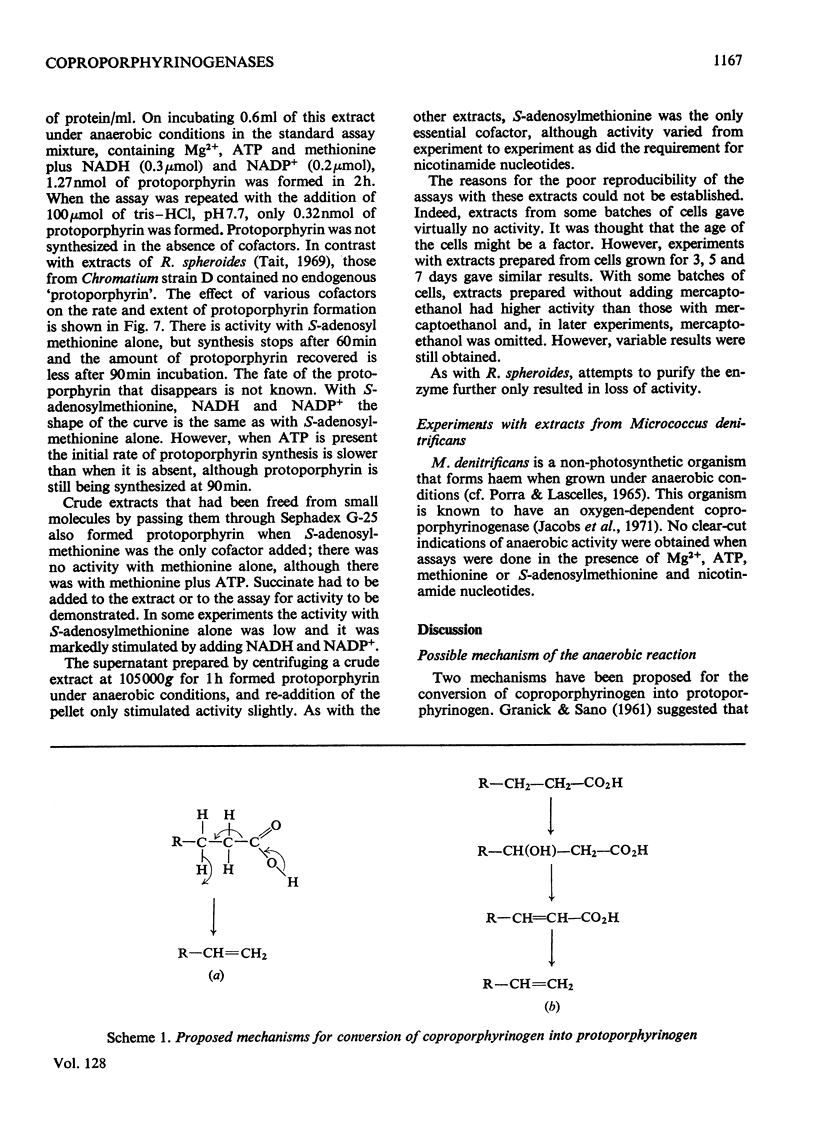
1. The anaerobic coproporphyrinogenase activity in an extract of Rhodopseudomonas spheroides is inhibited by 1,10-phenanthroline, αα′-bipyridyl, flavins, 2,4-dinitrophenol and 1,4-naphthaquinone. These compounds have no effect on the aerobic coproporphyrinogenase activity. 2. On removal of small-molecular-weight material from a crude extract, the anaerobic system becomes very unstable; it can be stabilized by adding succinate. Now nicotinamide nucleotides, in addition to Mg2+, ATP and methionine, are required for protoporphyrin to be formed. 3. A mechanism for the anaerobic reaction is proposed, based on the cofactor requirements and the effect of inhibitors. 4. The enzyme responsible for aerobic activity has been partially purified and some of its properties are reported. 5. A crude extract of Chromatium strain D also exhibits coproporphyrinogenase activity under anaerobic conditions in the presence of S-adenosylmethionine or ATP plus methionine. The requirement for other cofactors is variable.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANG J. P., MORRIS J. G. Studies on the utilization of nitrate by Micrococcus denitrificans. J Gen Microbiol. 1962 Oct;29:301–310. doi: 10.1099/00221287-29-2-301. [DOI] [PubMed] [Google Scholar]
- Chirpich T. P., Zappia V., Costilow R. N., Barker H. A. Lysine 2,3-aminomutase. Purification and properties of a pyridoxal phosphate and S-adenosylmethionine-activated enzyme. J Biol Chem. 1970 Apr 10;245(7):1778–1789. [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- Ehteshamuddin A. F. Anaerobic formation of protoporphyrin IX from coproporphyrinogen III by bacterial preparations. Biochem J. 1968 Apr;107(3):446–447. doi: 10.1042/bj1070446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Chapman J. R. Isolation of an hydroxylated porphyrin from urine and faeces of patients with cutaneous hepatic porphyria. Biochim Biophys Acta. 1970 Jun;208(3):535–537. doi: 10.1016/0304-4165(70)90229-1. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 1. The effect of growth conditions. Biochem J. 1962 Jun;83:539–549. doi: 10.1042/bj0830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 2. The effects of ethionine and threonine. Biochem J. 1962 Jun;83:550–559. doi: 10.1042/bj0830550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of prophyrin and bacteriochlorophyll by Rhodoseudomonas spheroides. 3. The effect of threonine on the biosynthesis of homoserine and methionine. Biochem J. 1962 Sep;84:483–490. doi: 10.1042/bj0840483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem J. 1972 Mar;127(1):97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W. P., Miller G. W. Coproporphyrinogenase in tobacco (Nicotiana tabacum L.). Biochem J. 1970 Apr;117(2):215–220. doi: 10.1042/bj1170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M., Brent P. Characterization of the late steps of microbial heme synthesis: conversion of coproporphyrinogen to protoporphyrin. J Bacteriol. 1971 Jul;107(1):203–209. doi: 10.1128/jb.107.1.203-209.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe J., Schacht J., Möckel W., Höpner T., Vetter H., Jr, Edenharder R. Pyruvate formate-lyase reaction in Escherichia coli. The enzymatic system converting an inactive form of the lyase into the catalytically active enzyme. Eur J Biochem. 1969 Dec;11(2):316–327. doi: 10.1111/j.1432-1033.1969.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Knobloch K., Eley J. H., Aleem M. I. Generation of reducing power in bacterial photosynthesis. Rhodopseudomonas palustris. Biochem Biophys Res Commun. 1971 May 21;43(4):834–839. doi: 10.1016/0006-291x(71)90692-9. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mori M., Sano S. Protoporphyrin formation from coproporphyrinogen III by Chromatium cell extracts. Biochem Biophys Res Commun. 1968 Aug 21;32(4):610–615. doi: 10.1016/0006-291x(68)90281-7. [DOI] [PubMed] [Google Scholar]
- Morita S., Edwards M., Gibson J. Influence of metabolic conditions on light-induced absorbancy changes in Chromatium D. Biochim Biophys Acta. 1965 Sep 27;109(1):45–58. doi: 10.1016/0926-6585(65)90089-0. [DOI] [PubMed] [Google Scholar]
- NISHIDA G., LABBE R. F. Heme biosynthesis; on the incorporation of iron into protoporphyrin. Biochim Biophys Acta. 1959 Feb;31(2):519–524. doi: 10.1016/0006-3002(59)90028-9. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., LASCELLES J. HAEMOPROTEINS AND HAEM SYNTHESIS IN FACULTATIVE PHOTOSYNTHETIC AND DENITRIFYING BACTERIA. Biochem J. 1965 Jan;94:120–126. doi: 10.1042/bj0940120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulland-Dussoix D., Boyer H. W. The Escherichia coli B restriction endonuclease. Biochim Biophys Acta. 1969 Nov 19;195(1):219–229. doi: 10.1016/0005-2787(69)90618-2. [DOI] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Sano S. 2,4-Bis-(beta-hydroxypropionic acid) deuteroporphyrinogen IX, a possible intermediate between coproporphyrinogen 3 and protoporphyrin IX. J Biol Chem. 1966 Nov 25;241(22):5276–5283. [PubMed] [Google Scholar]
- Scholes P. B., Smith L. The isolation and properties of the cytoplasmic membrane of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):350–362. doi: 10.1016/0005-2728(68)90080-7. [DOI] [PubMed] [Google Scholar]
- Sewell D. L., Aleem M. I. Generation of reducing power in chemosynthesis. V. The mechanism of pyridine nucleotide reduction by nitrite in the chemoautotroph Nitrobacter agilis. Biochim Biophys Acta. 1969 Apr 8;172(3):467–475. doi: 10.1016/0005-2728(69)90143-1. [DOI] [PubMed] [Google Scholar]
- Tait G. H. Coproporphyrinogenase activity in extracts from Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1969 Sep 24;37(1):116–122. doi: 10.1016/0006-291x(69)90888-2. [DOI] [PubMed] [Google Scholar]
- del Batlle A. M., Benson A., Rimington C. Purification and properties of coproporphyrinogenase. Biochem J. 1965 Dec;97(3):731–740. doi: 10.1042/bj0970731. [DOI] [PMC free article] [PubMed] [Google Scholar]


