Abstract
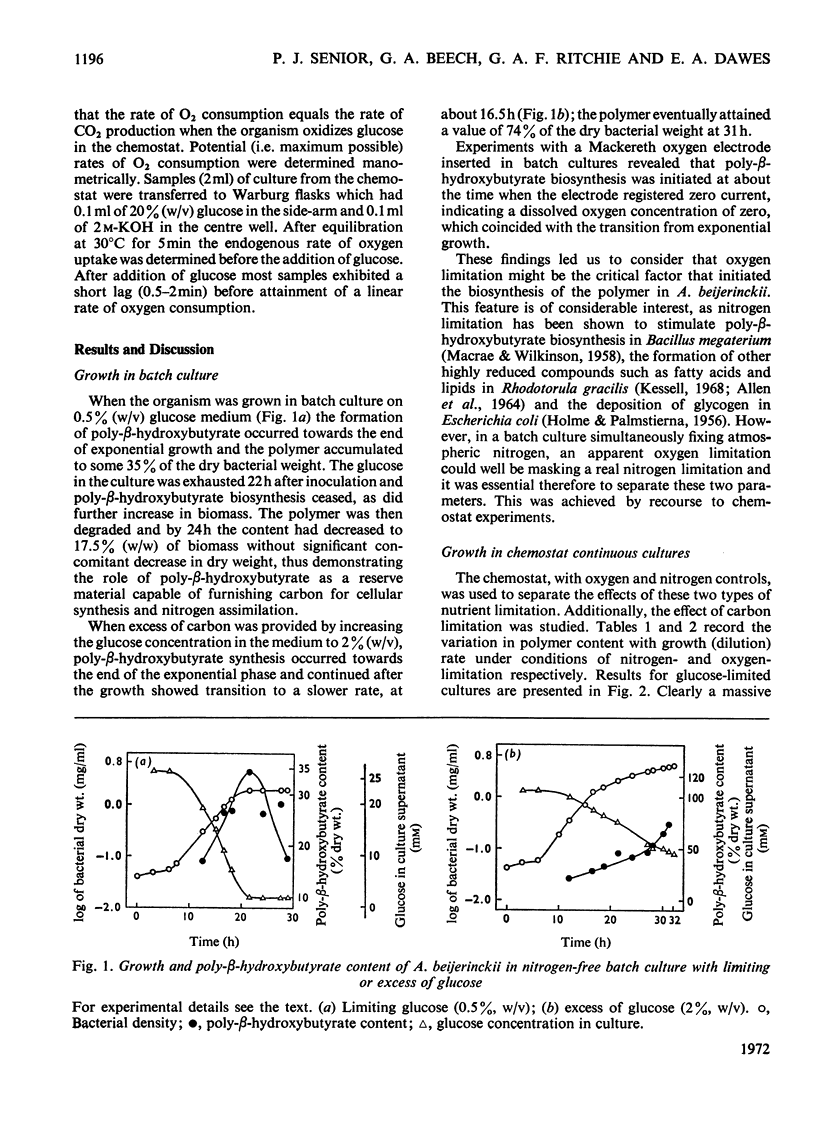
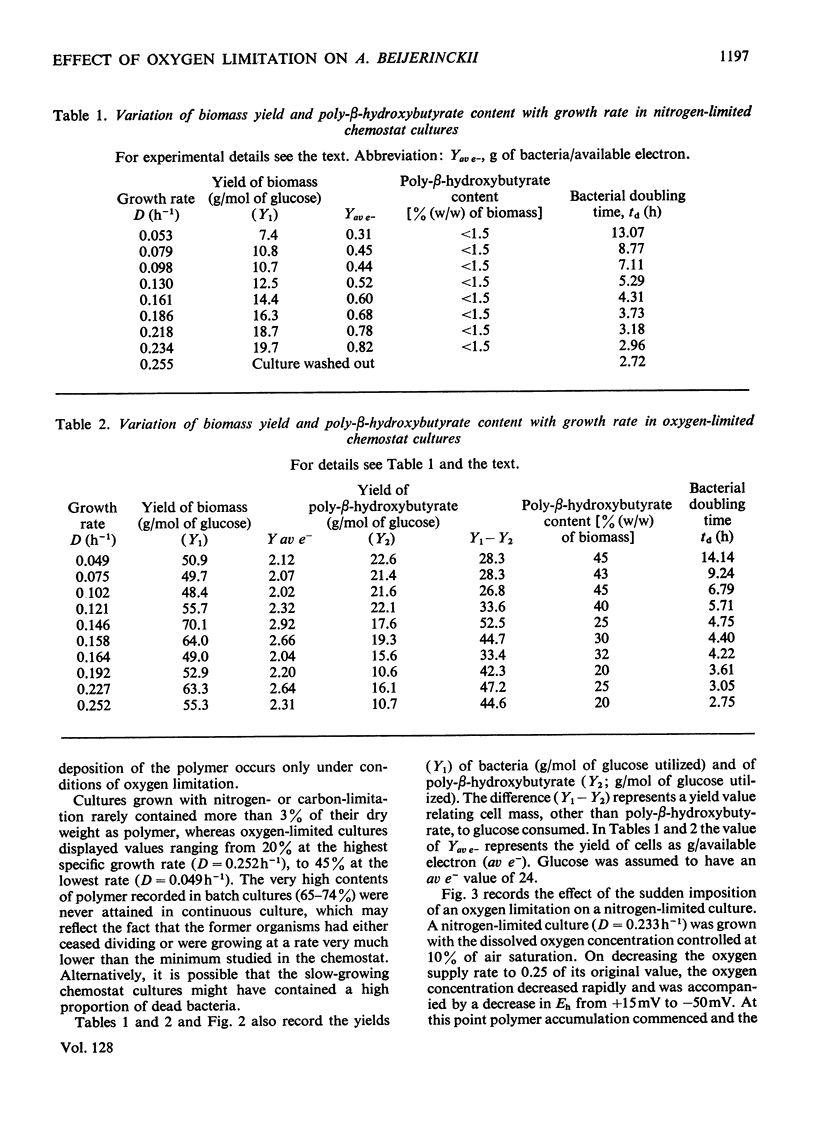
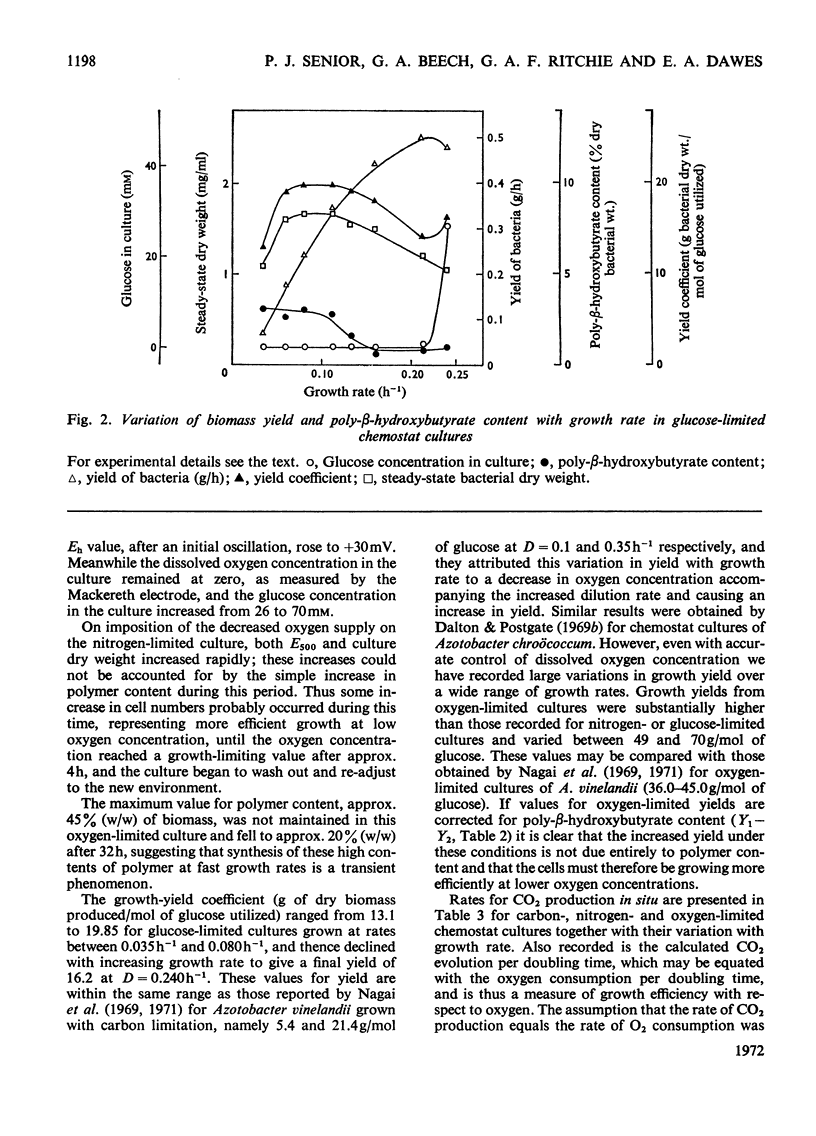
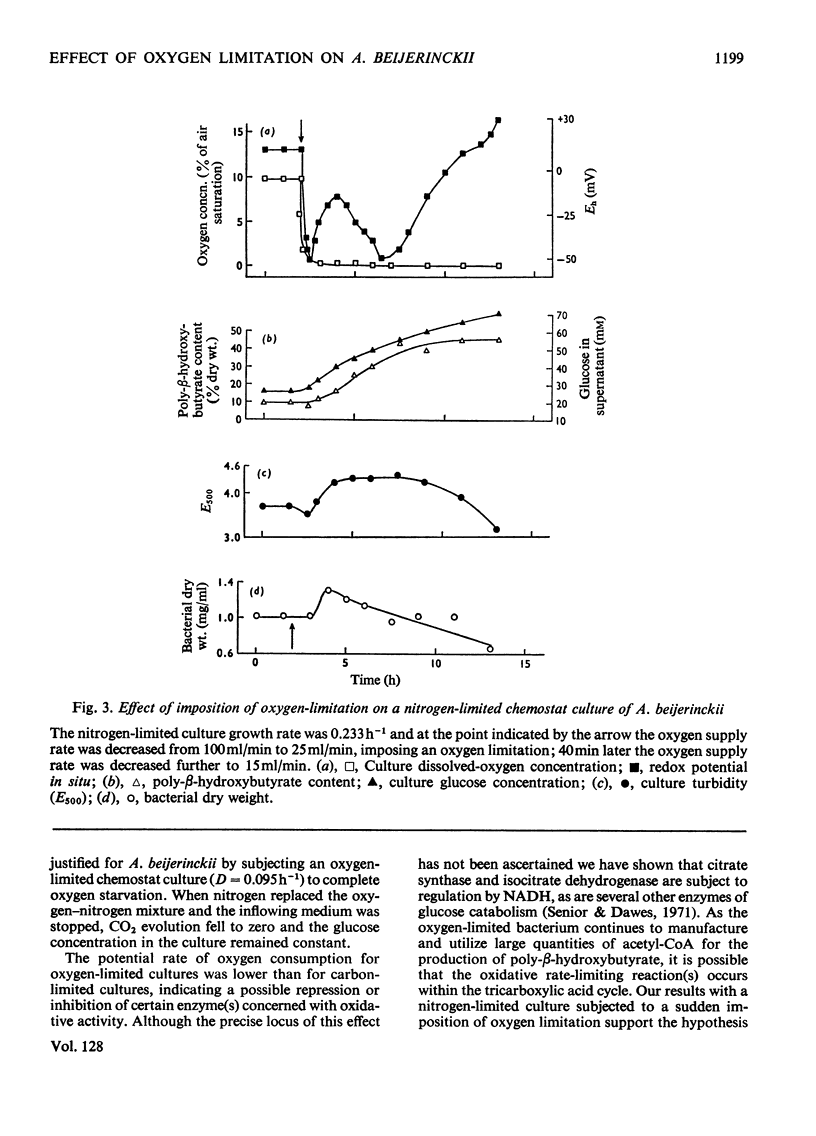
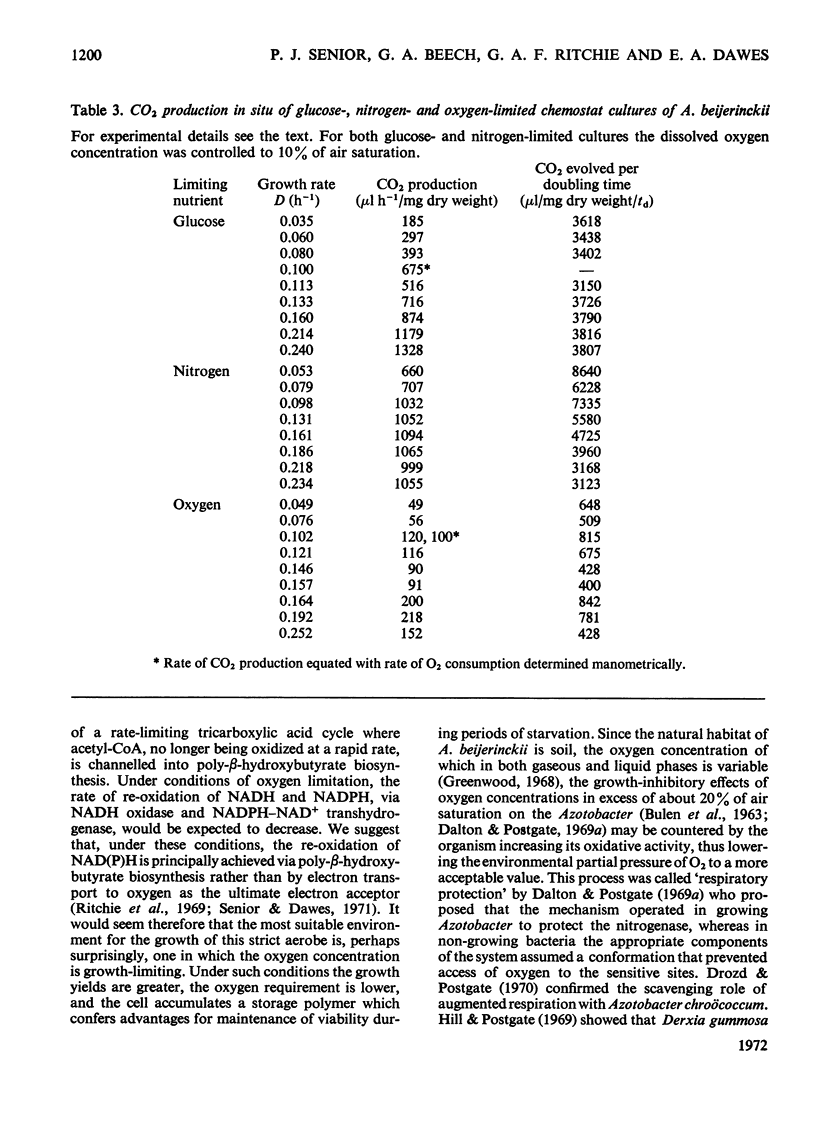
Azotobacter beijerinckii was grown in ammonia-free glucose–mineral salts media in batch culture and in chemostat cultures limited by the supply of glucose, oxygen or molecular nitrogen. In batch culture poly-β-hydroxybutyrate was formed towards the end of exponential growth and accumulated to about 74% of the cell dry weight. In chemostat cultures little poly-β-hydroxybutyrate accumulated in organisms that were nitrogen-limited, but when oxygen limited a much increased yield of cells per mol of glucose was observed, and the organisms contained up to 50% of their dry weight of poly-β-hydroxybutyrate. In carbon-limited cultures (D, the dilution rate,=0.035–0.240h−1), the growth yield ranged from 13.1 to 19.8g/mol of glucose and the poly-β-hydroxybutyrate content did not exceed 3.0% of the dry weight. In oxygen-limited cultures (D=0.049–0.252h−1) the growth yield ranged from 48.4 to 70.1g/mol of glucose and the poly-β-hydroxybutyrate content was between 19.6 and 44.6% of dry weight. In nitrogen-limited cultures (D=0.053–0.255h−1) the growth yield ranged from 7.45 to 19.9g/mol of glucose and the poly-β-hydroxybutyrate content was less than 1.5% of dry weight. The sudden imposition of oxygen limitation on a nitrogen-limited chemostat culture produced a rapid increase in poly-β-hydroxybutyrate content and cell yield. Determinations on chemostat cultures revealed that during oxygen-limited steady states (D=0.1h−1) the oxygen uptake decreased to 100μl h−1 per mg dry wt. compared with 675 for a glucose-limited culture (D=0.1h−1). Nitrogen-limited cultures had CO2 production values in situ ranging from 660 to 1055μl h−1 per mg dry wt. at growth rates of 0.053–0.234h−1 and carbon-limited cultures exhibited a variation of CO2 production between 185 and 1328μl h−1 per mg dry wt. at growth rates between 0.035 and 0.240h−1. These findings are discussed in relation to poly-β-hydroxybutyrate formation, growth efficiency and growth yield during growth on glucose. We suggest that poly-β-hydroxybutyrate is produced in response to oxygen limitation and represents not only a store of carbon and energy but also an electron sink into which excess of reducing power can be channelled.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A., LECOMTE J. R., BALES H. E. SHORT-TERM N2-15 INCORPORATION BY AZOBACTER. J Bacteriol. 1963 Mar;85:666–670. doi: 10.1128/jb.85.3.666-670.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Drozd J., Postgate J. R. Effects of oxygen on acetylene reduction, cytochrome content and respiratory activity of Azotobacter chroococcum. J Gen Microbiol. 1970 Sep;63(1):63–73. doi: 10.1099/00221287-63-1-63. [DOI] [PubMed] [Google Scholar]
- Herbert D., Phipps P. J., Tempest D. W. The chemostat: design and instrumentation. Lab Pract. 1965 Oct;14(10):1150–1161. [PubMed] [Google Scholar]
- Hill S., Postgate J. R. Failure of putative nitrogen-fixing bacteria to fix nitrogen. J Gen Microbiol. 1969 Oct;58(2):277–285. doi: 10.1099/00221287-58-2-277. [DOI] [PubMed] [Google Scholar]
- JENSEN H. L. The Azotobacteriaceae. Bacteriol Rev. 1954 Dec;18(4):195–214. doi: 10.1128/br.18.4.195-214.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessell R. H. Fatty acids of Rhodotorula gracilis: fat production in submerged culture and the particular effect of pH value. J Appl Bacteriol. 1968 Jun;31(2):220–231. doi: 10.1111/j.1365-2672.1968.tb00361.x. [DOI] [PubMed] [Google Scholar]
- MACRAE R. M., WILKINSON J. F. Poly-beta-hyroxybutyrate metabolism in washed suspensions of Bacillus cereus and Bacillus megaterium. J Gen Microbiol. 1958 Aug;19(1):210–222. doi: 10.1099/00221287-19-1-210. [DOI] [PubMed] [Google Scholar]
- MacLennan D. G., Pirt S. J. Automatic control of dissolved oxygen concentration in stirred microbial cultures. J Gen Microbiol. 1966 Nov;45(2):289–302. doi: 10.1099/00221287-45-2-289. [DOI] [PubMed] [Google Scholar]
- Nagai S., Nishizawa Y., Aiba S. Energetics of growth of Azotobacter vinelandii in a glucose-limited chemostat culture. J Gen Microbiol. 1969 Dec;59(2):163–169. doi: 10.1099/00221287-59-2-163. [DOI] [PubMed] [Google Scholar]
- Nagai S., Nishizawa Y., Onodera M., Aiba S. Effect of dissolved oxygen on growth yield and aldolase activity in chemostat culture of Azotobacter vinelandii. J Gen Microbiol. 1971 May;66(2):197–203. doi: 10.1099/00221287-66-2-197. [DOI] [PubMed] [Google Scholar]
- Ritchie G. A., Dawes E. A. The non-involvement of cyl-carrir protein in poly-beta-hydroxybutyric acid biosynthesis in Azotobacter beijerinckii. Biochem J. 1969 May;112(5):803–805. doi: 10.1042/bj1120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie G. A., Senior P. J., Dawes E. A. The purification and characterization of acetoacetyl-coenzyme A reductase from Azotobacter beijerinckii. Biochem J. 1971 Jan;121(2):309–316. doi: 10.1042/bj1210309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. Poly- -hydroxybutyrate biosynthesis and the regulation of glucose metabolism in Azotobacter beijerinckii. Biochem J. 1971 Nov;125(1):55–66. doi: 10.1042/bj1250055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale H., Ribbons D. W., Dawes E. A. Occurrence of poly-beta-hydroxybutyrate in the Azotobacteriaceae. J Bacteriol. 1968 May;95(5):1798–1803. doi: 10.1128/jb.95.5.1798-1803.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., WILKINSON J. F. The isolation and estimation of the poly-beta-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol. 1958 Aug;19(1):198–209. doi: 10.1099/00221287-19-1-198. [DOI] [PubMed] [Google Scholar]


