Abstract
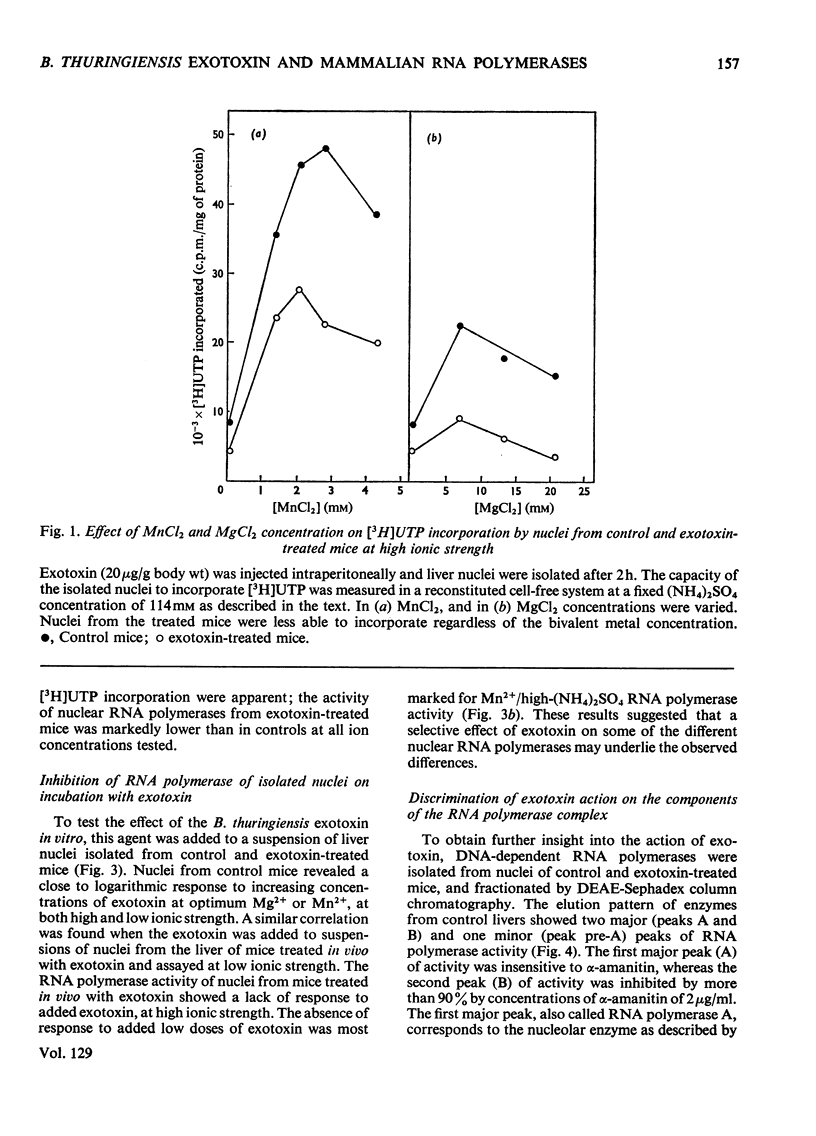
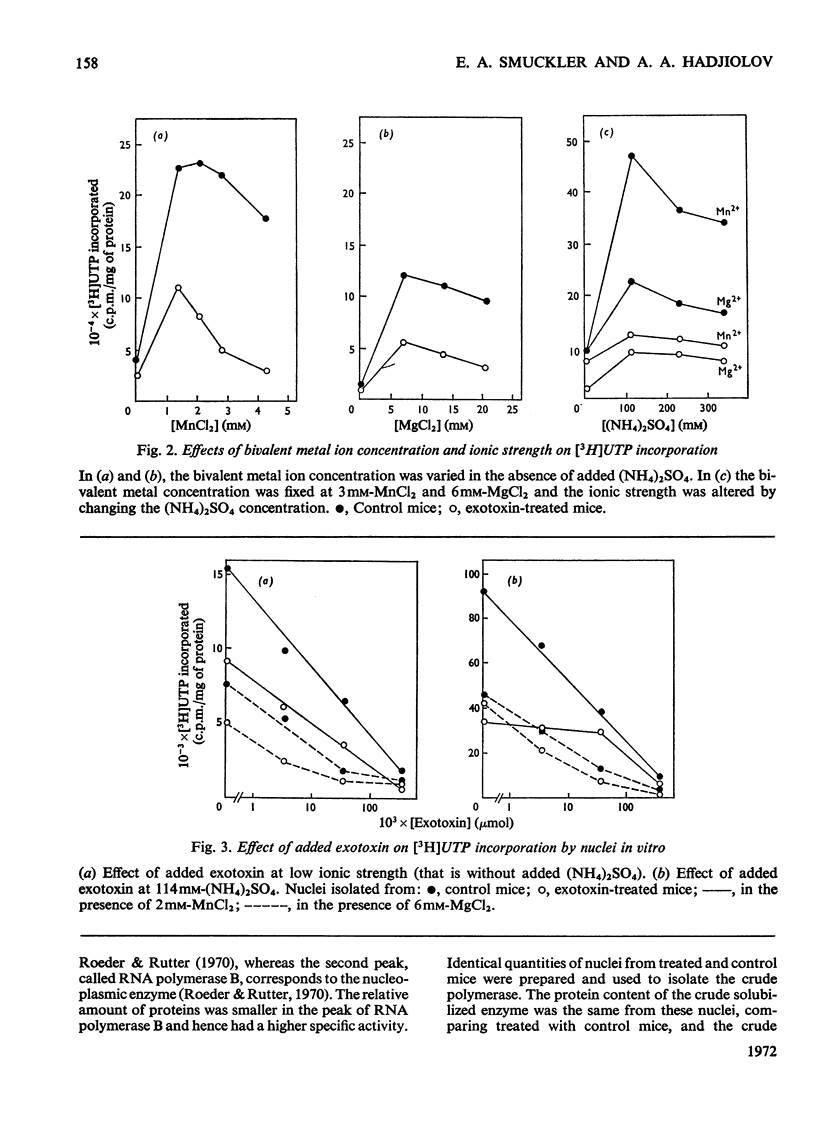
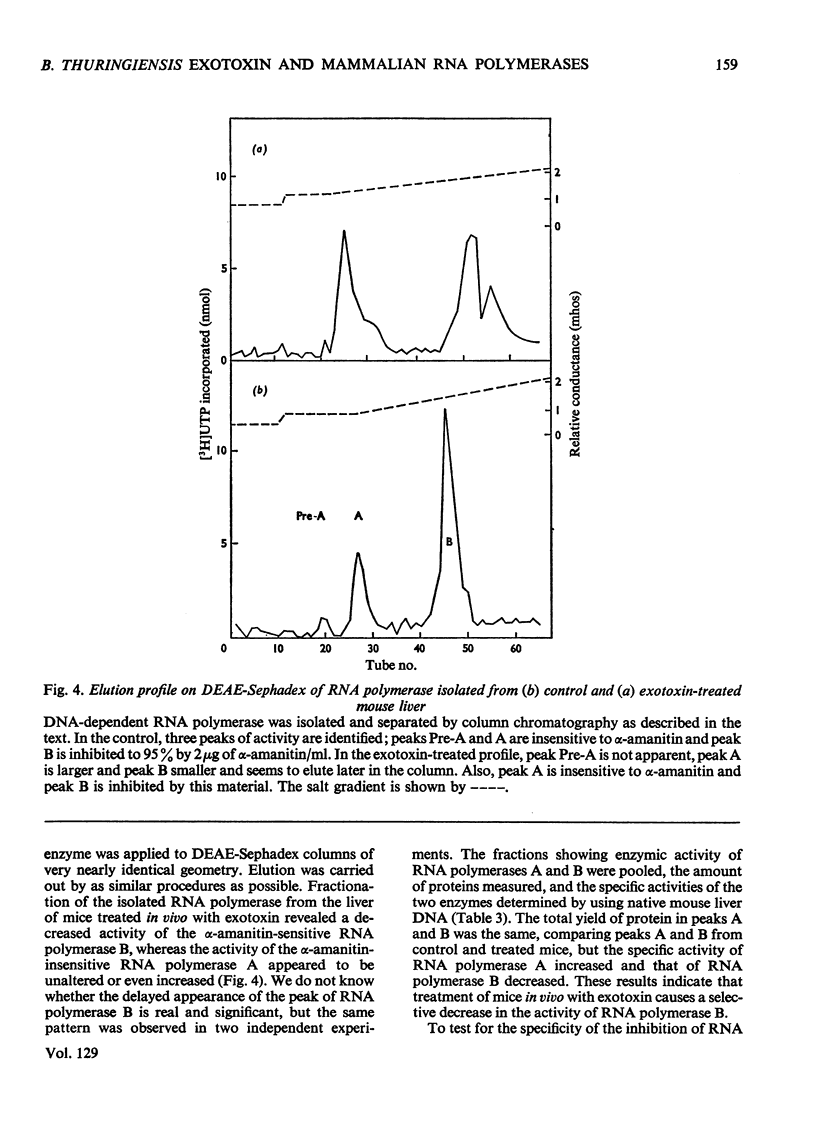
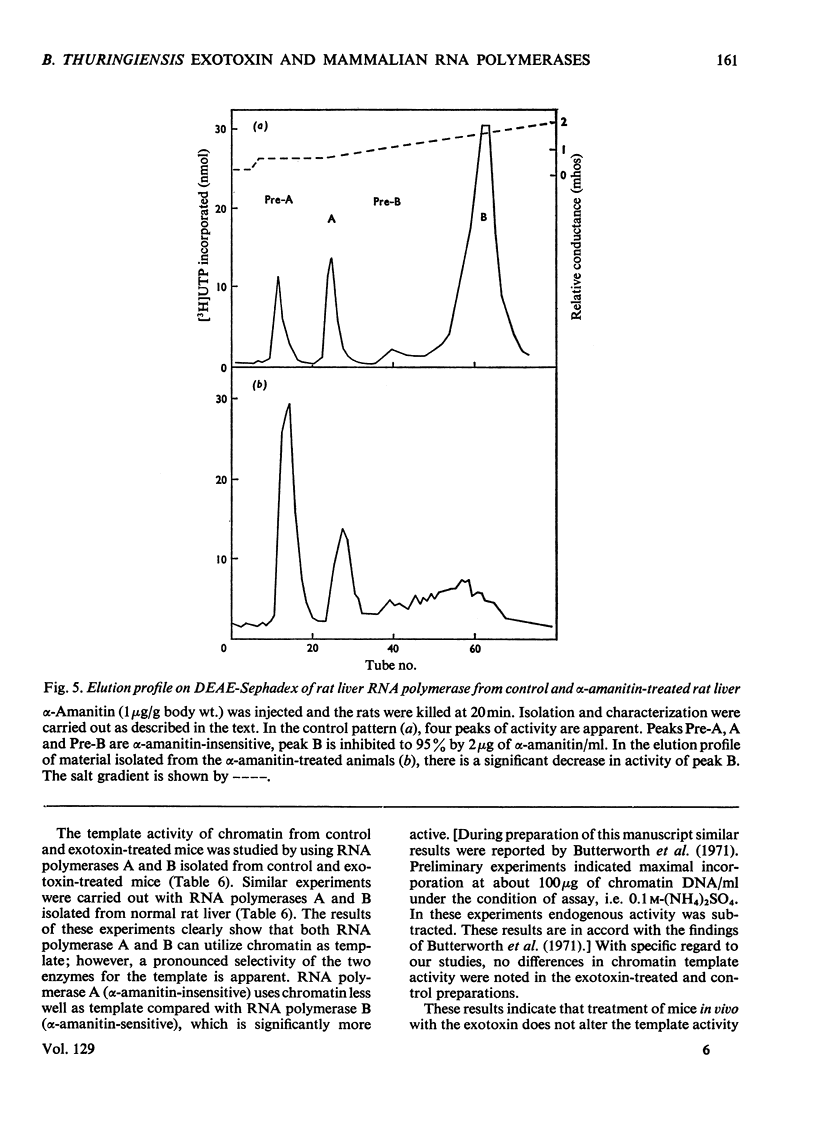
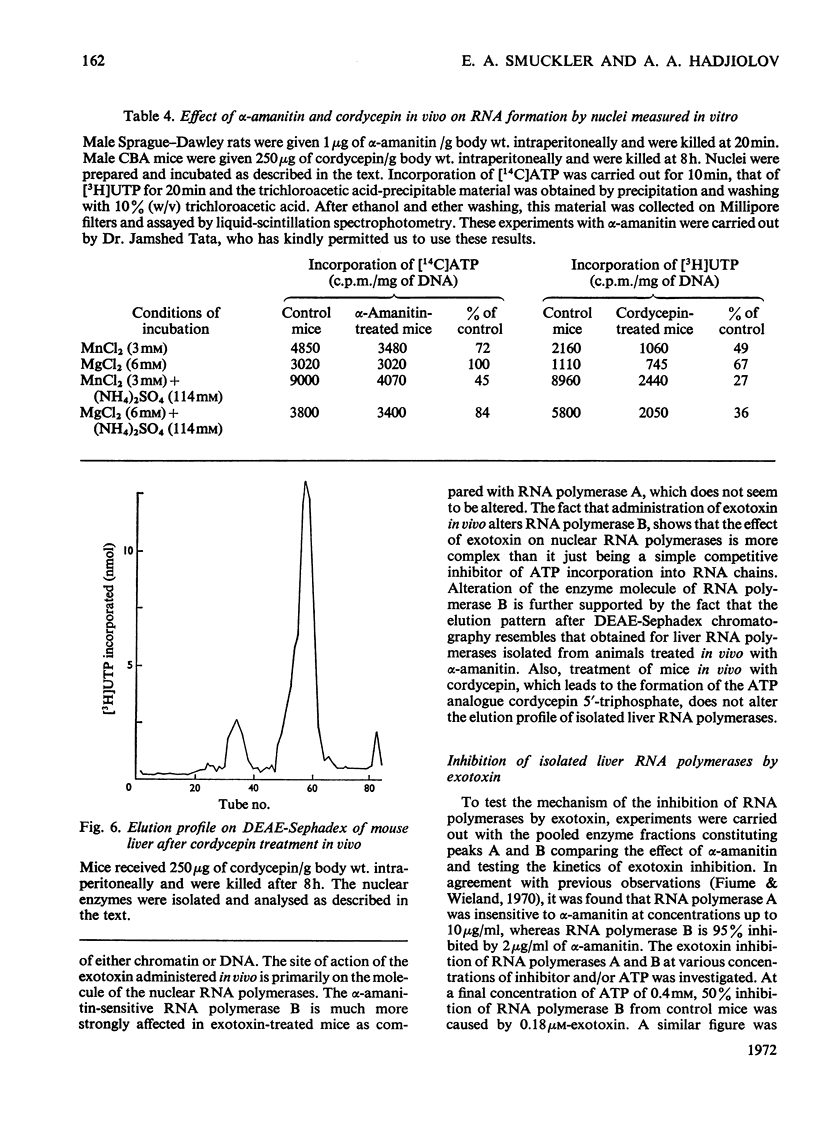
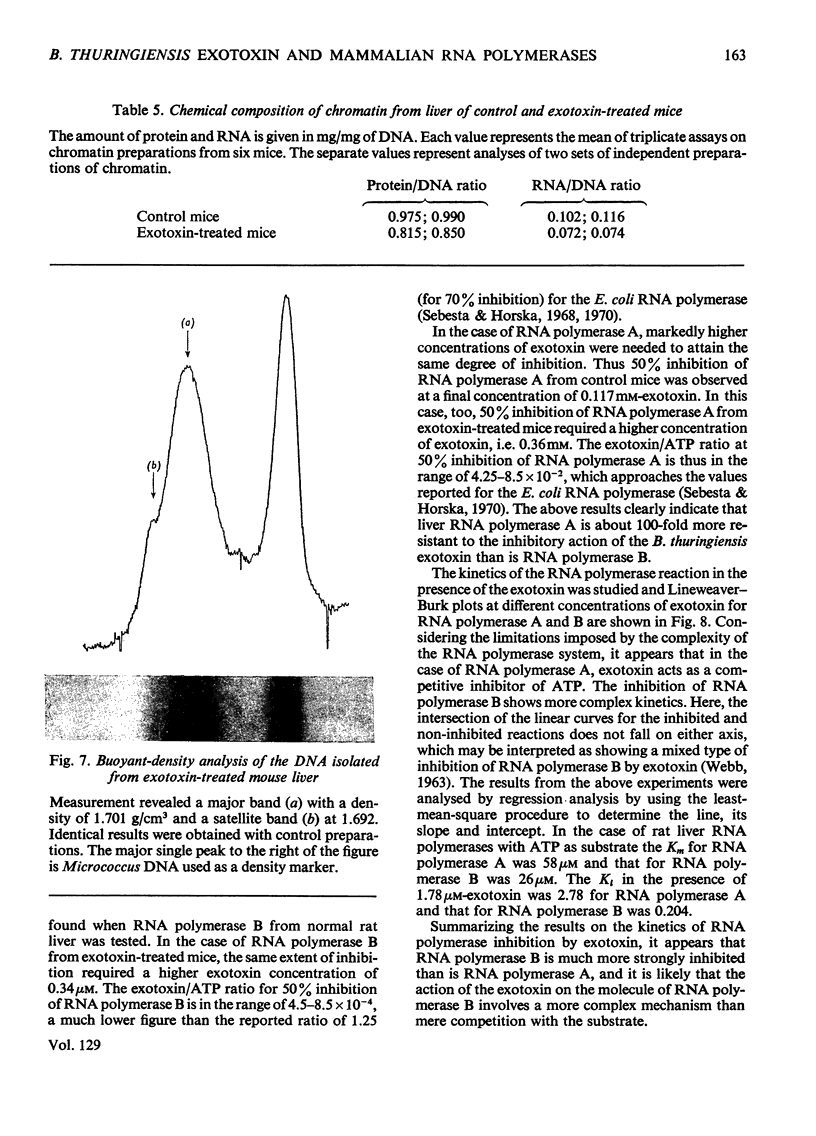
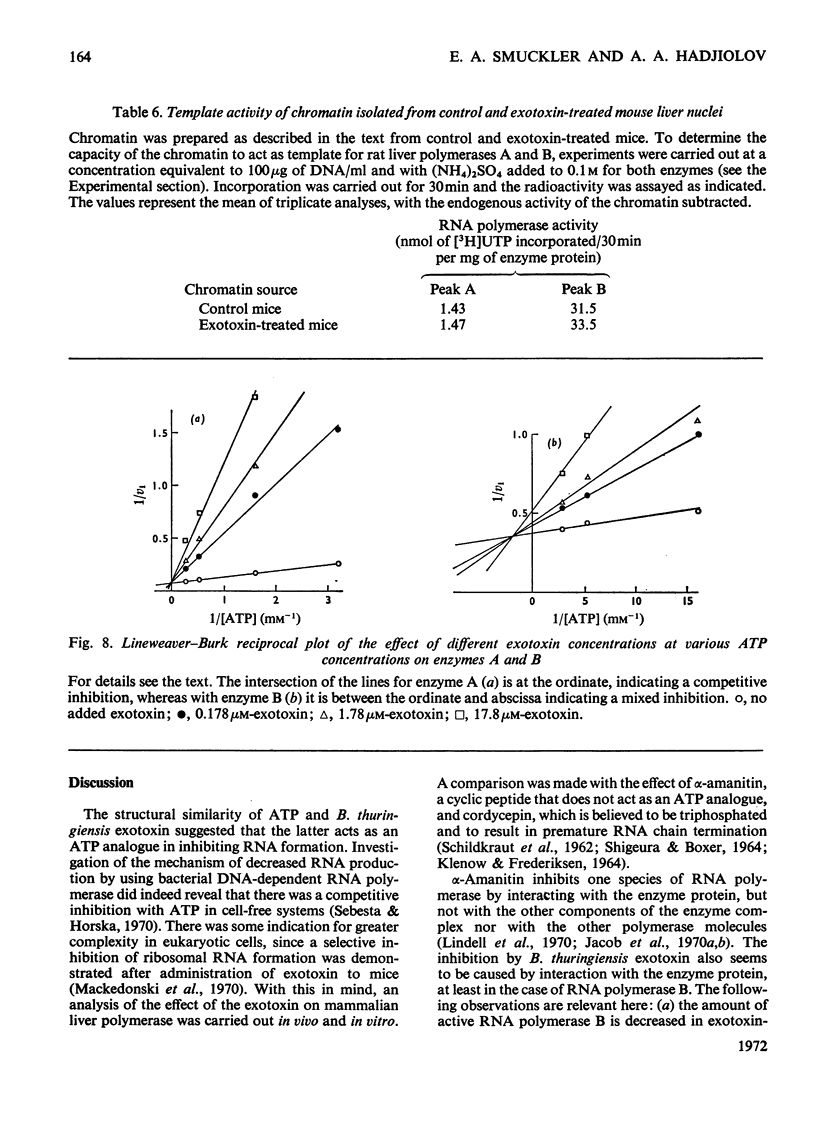
The action of Bacillus thuringiensis exotoxin, a structural analogue of ATP, on mouse liver DNA-dependent RNA polymerases was studied and its effects were compared with those of α-amanitin and cordycepin. (1) Administration of exotoxin in vivo caused a marked decrease in RNA polymerase activity of isolated nuclei at various concentrations of Mg2+, Mn2+ and (NH4)2SO4. A similar action was recorded after addition of exotoxin to isolated nuclei from control or exotoxin-treated mice. (2) Chromatographic separation of nuclear RNA polymerases from mice treated in vivo with exotoxin showed a drastic decrease of the peak of nucleoplasmic RNA polymerase, whereas the peak of nucleolar RNA polymerase remained unaltered. The same effect was observed after administration of α-amanitin in vivo, but cordycepin did not alter the relative amounts of the two main RNA polymerase peaks. (3) Administration of exotoxin in vivo did not alter the template activity of isolated DNA or chromatin tested with different fractions of RNA polymerase from control or exotoxin-treated mice. (4) Addition of exotoxin to isolated liver RNA polymerases inhibited both enzyme fractions. However, the α-amanitin-sensitive RNA polymerase was also 50–100-fold more sensitive to exotoxin inhibition than was the α-amanitin-insensitive RNA polymerase. Kinetic analysis indicated the exotoxin produces a competitive inhibition with ATP on the nucleolar enzyme, but a mixed type of inhibition with nucleoplasmic enzyme. The results obtained indicate that the B. thuringiensis exotoxin inhibits liver RNA synthesis by affecting nuclear RNA polymerases, showing a preferential inhibition of the nucleoplasmic α-amanitin-sensitive RNA polymerase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Bond R. P., Boyce C. B., French S. J. A purification and some properties of an insecticidal exotoxin from Bacillus thuringiensis Berliner. Biochem J. 1969 Sep;114(3):477–488. doi: 10.1042/bj1140477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Butterworth P. H., Cox R. F., Chesterton C. J. Transcription of mammalian chromatin by mammalian DNA-dependent RNA polymerases. Eur J Biochem. 1971 Nov 11;23(2):229–241. doi: 10.1111/j.1432-1033.1971.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Butterworth P. H.W. A new form of mammalian DNA-dependent RNA polymerase and its relationship to the known forms of the enzyme. FEBS Lett. 1971 Feb 9;12(6):301–308. doi: 10.1016/0014-5793(71)80001-7. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Muecke W., Sajdel E. M., Munro H. N. Evidence for extranucleolar control of RNA synthesis in the nucleolus. Biochem Biophys Res Commun. 1970 Jul 27;40(2):334–342. doi: 10.1016/0006-291x(70)91014-4. [DOI] [PubMed] [Google Scholar]
- KIT S. Equilibrium sedimentation in density gradients of DNA preparations from animal tissues. J Mol Biol. 1961 Dec;3:711–716. doi: 10.1016/s0022-2836(61)80075-2. [DOI] [PubMed] [Google Scholar]
- KLENOW H., FREDERIKSEN S. EFFECT OF 3'-DEOXYATP (CORDYCEPIN TRIPHOSPHATE) AND 2'-DEOXYATP ON THE DNA-DEPENDENT RNA NEUCLEOTIDYLTRANSFERASE FROM EHRLICH ASCITES TUMOR CELLS. Biochim Biophys Acta. 1964 Jul 22;87:495–498. doi: 10.1016/0926-6550(64)90122-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sebesta K., Horská K. Inhibition of DNA-dependent RNA polymerase by the exotosin of Bacillus thuringiensis var. gelechiae. Biochim Biophys Acta. 1968 Nov 20;169(1):281–282. doi: 10.1016/0005-2787(68)90035-x. [DOI] [PubMed] [Google Scholar]
- Sebesta K., Horská K. Mechanism of inhibition of DNA-dependent RNA polymerase by exotoxin of Bacillus thuringiensis. Biochim Biophys Acta. 1970;209(2):357–376. doi: 10.1016/0005-2787(70)90734-3. [DOI] [PubMed] [Google Scholar]
- Siev M., Weinberg R., Penman S. The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin. J Cell Biol. 1969 May;41(2):510–520. doi: 10.1083/jcb.41.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuckler E. A., Tata J. R. Changes in hepatic nuclear DNA-dependent RNA polymerase caused by growth hormone and triiodothyronine. Nature. 1971 Nov 5;234(5323):37–39. doi: 10.1038/234037a0. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. Studies on the stimulation by ammonium sulphate of the DNA-dependent RNA polymerase of isolated rat-liver nuclei. Biochim Biophys Acta. 1966 Sep;123(3):478–492. doi: 10.1016/0005-2787(66)90216-4. [DOI] [PubMed] [Google Scholar]
- de Barjac H., Dedonder R. Purification de la toxine thermostable de B. thuringiensis var. thuringiensis et analyses complémentaires. Bull Soc Chim Biol (Paris) 1968;50(4):941–944. [PubMed] [Google Scholar]