Abstract
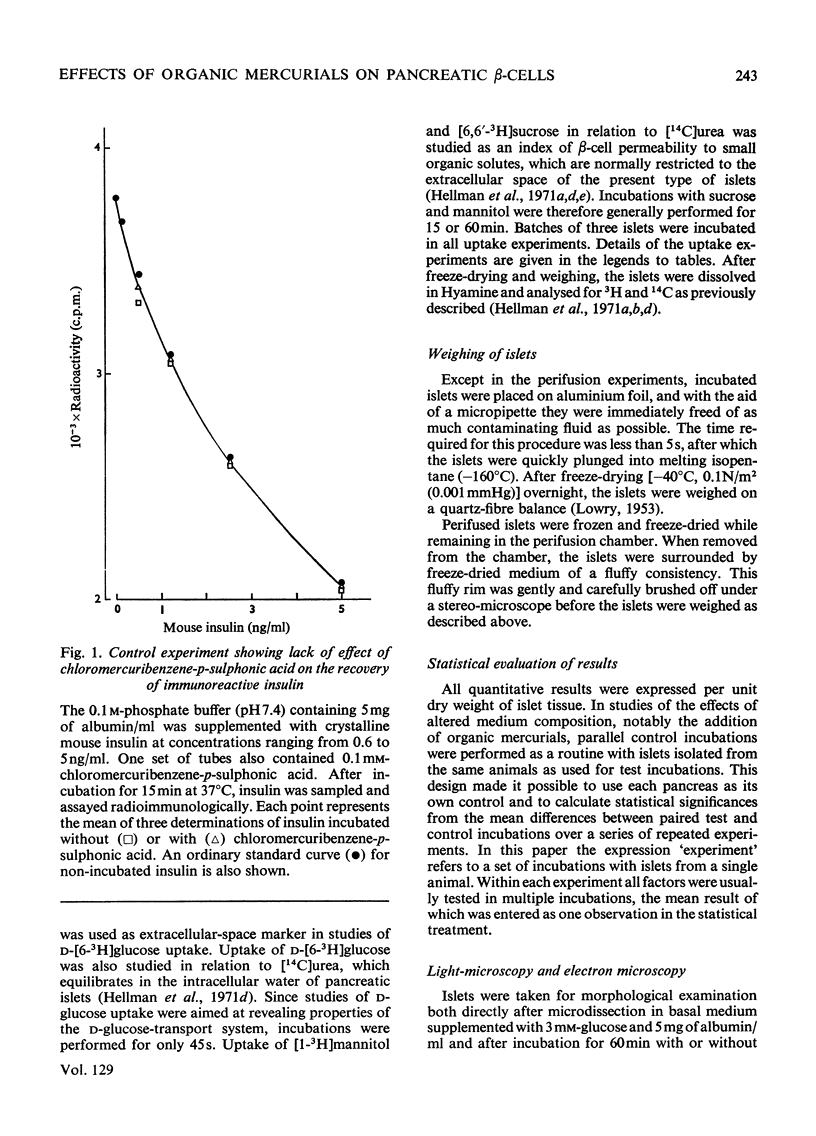
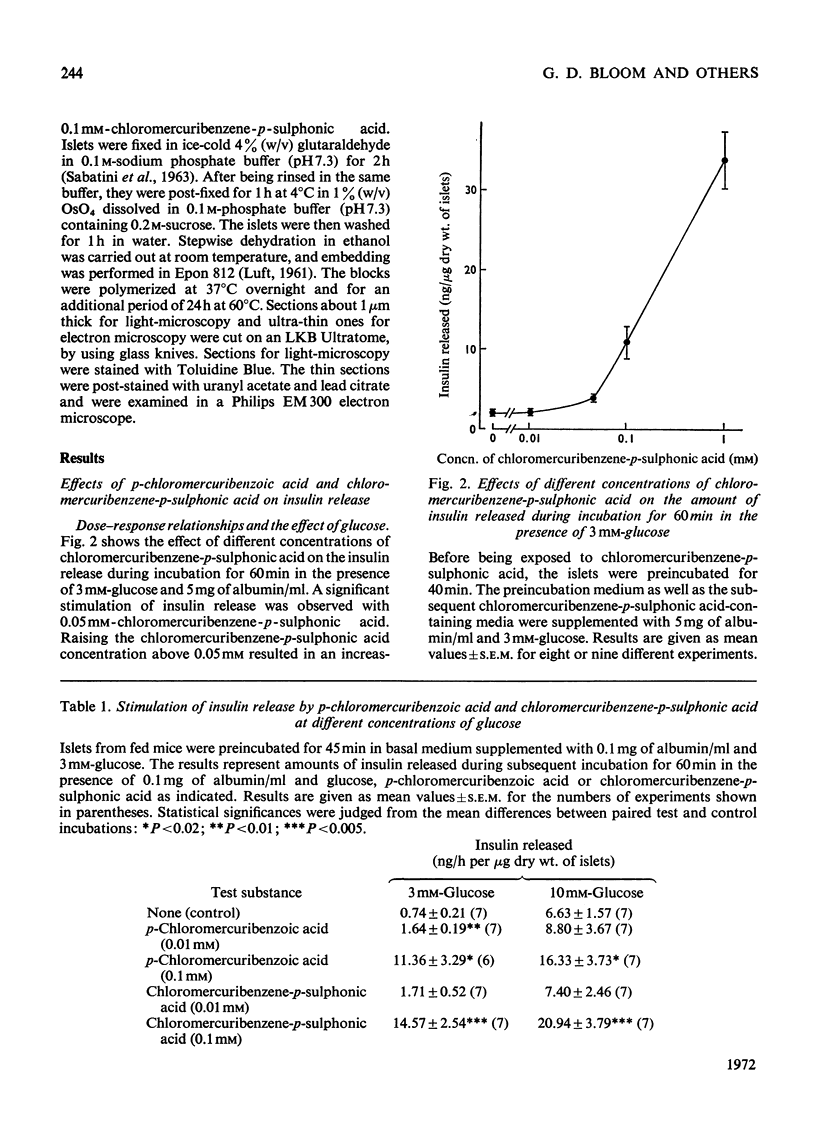
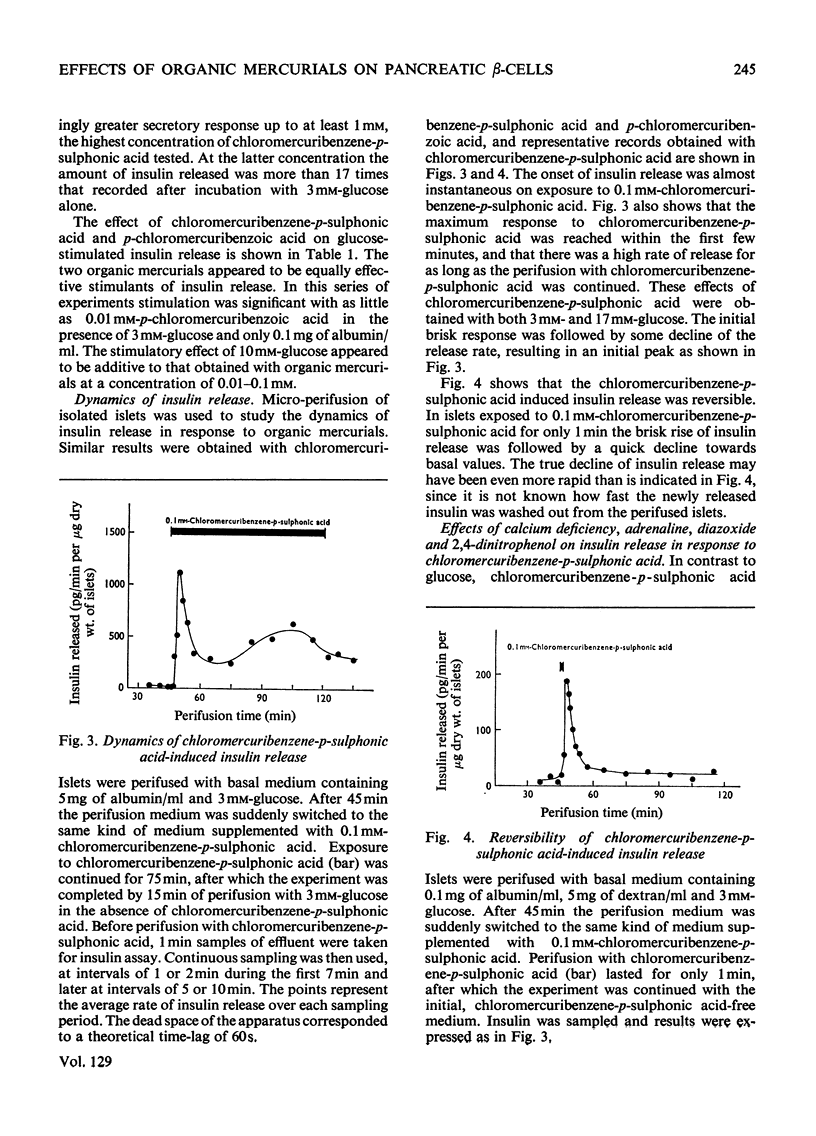
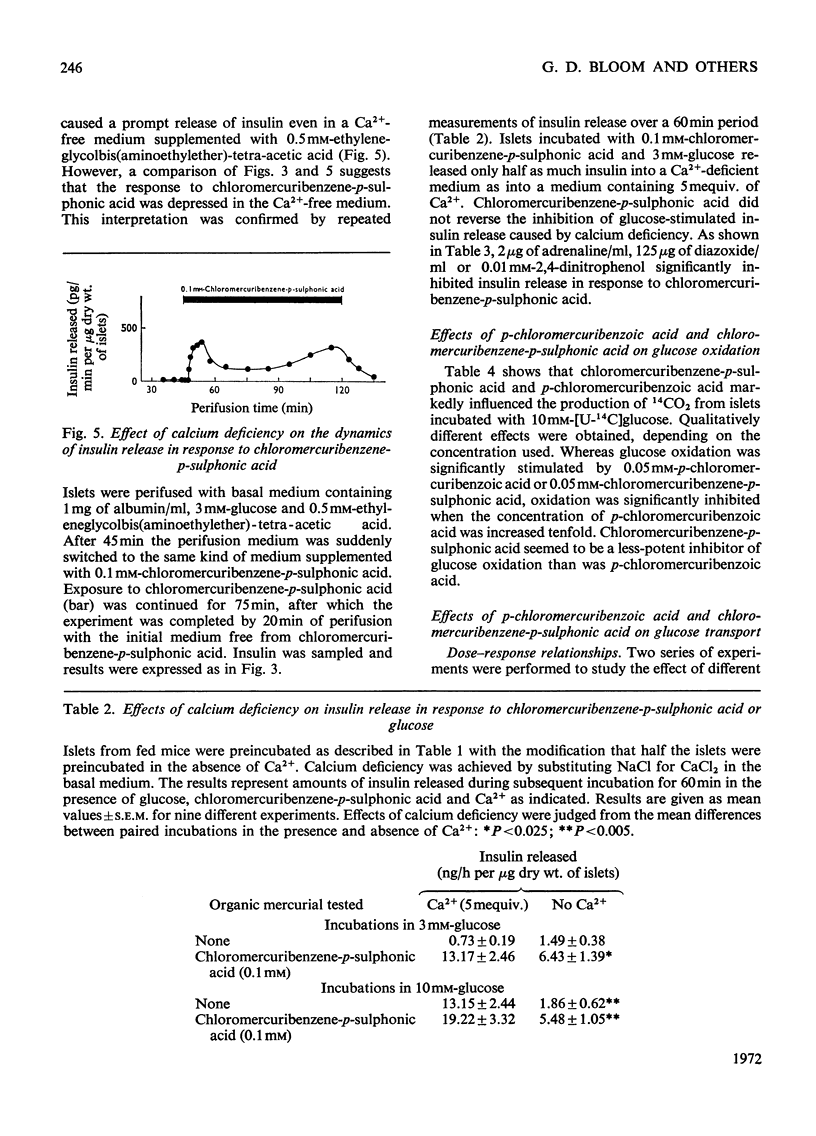
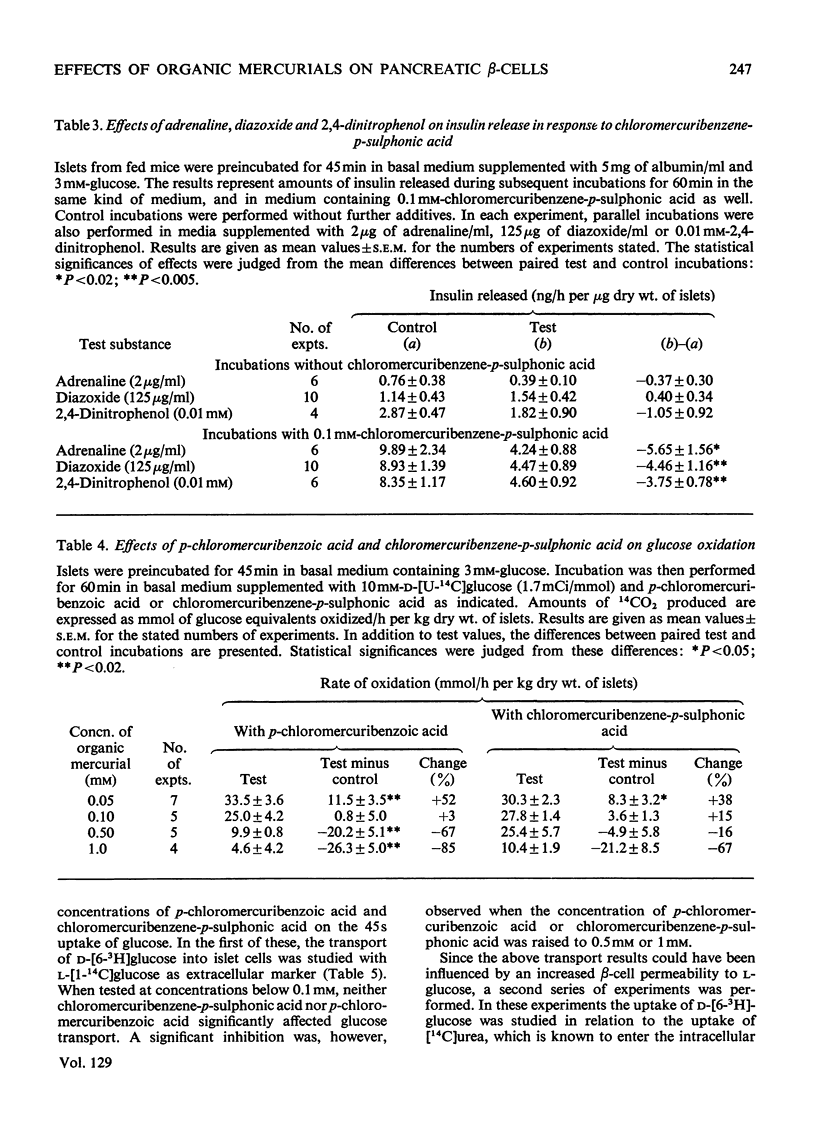
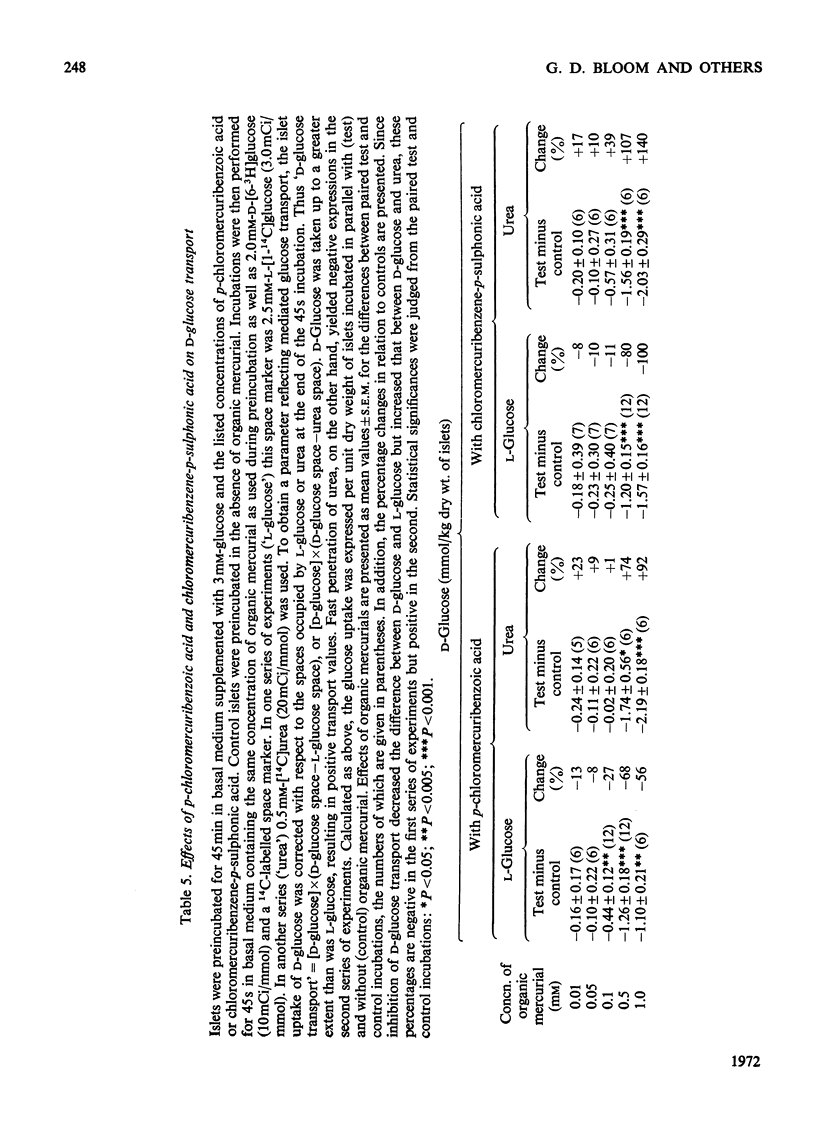
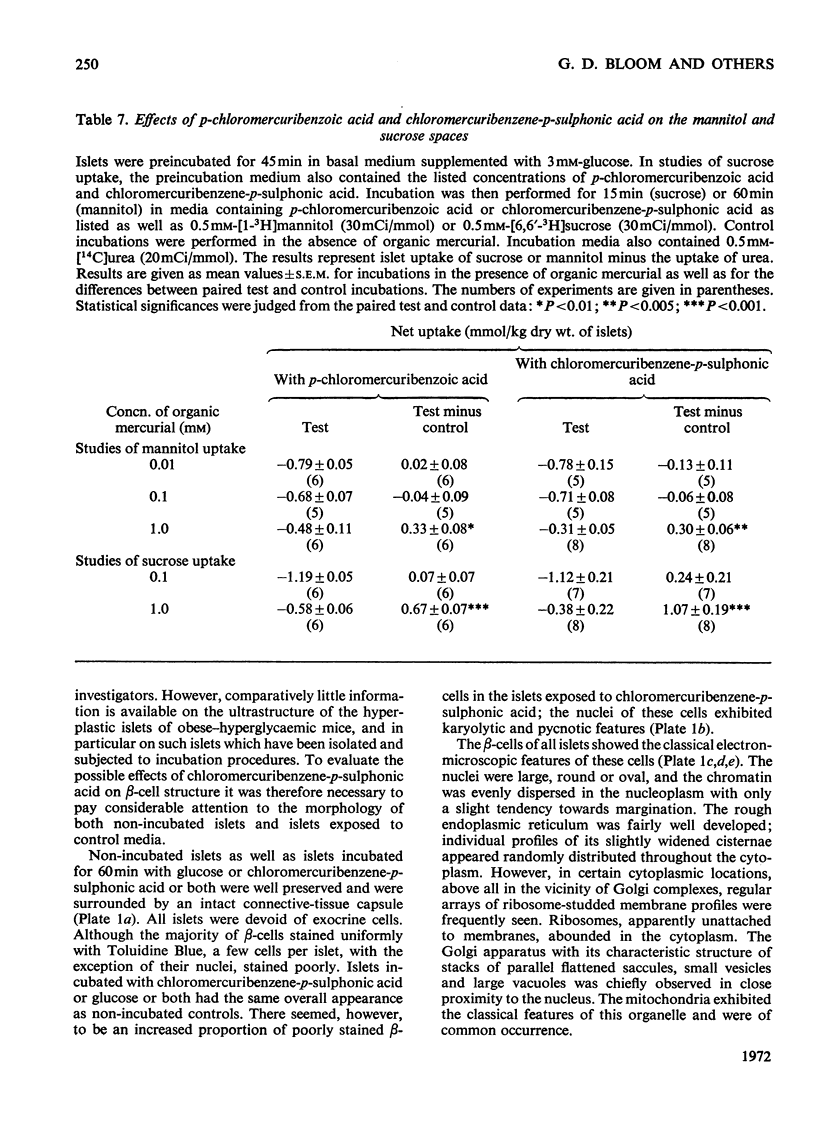
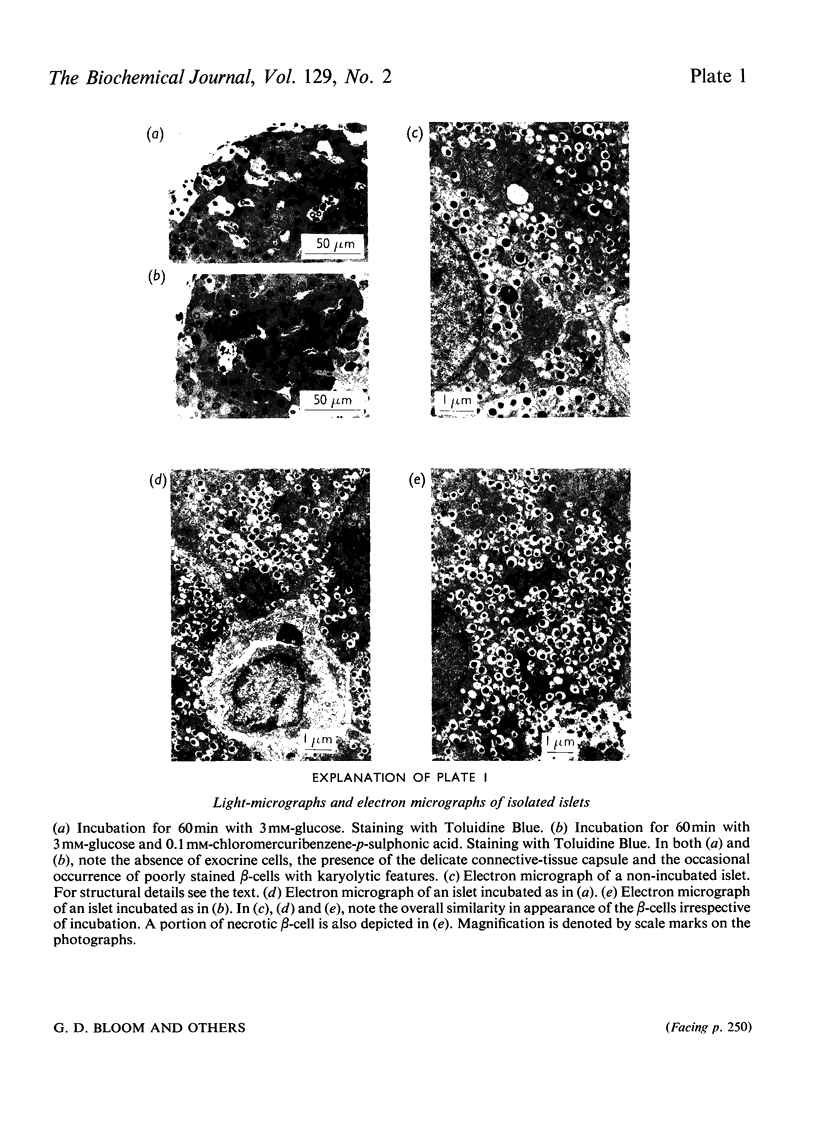
The effects of p-chloromercuribenzoic acid and chloromercuribenzene-p-sulphonic acid on pancreatic islets were studied in vitro. Obese–hyperglycaemic mice were used as the source of microdissected islets containing more than 90% β-cells. p-Chloromercuribenzoic acid and chloromercuribenzene-p-sulphonic acid stimulated insulin release at concentrations of 0.01mm or above. This stimulation was significantly inhibited by the omission of Ca2+ or the addition of adrenaline, diazoxide or 2,4-dinitrophenol. p-Chloromercuribenzoic acid or chloromercuribenzene-p-sulphonic acid did not interfere with the insulin-releasing ability of glucose. Micro-perifusion experiments revealed that the release of insulin in response to organic mercurial occurred almost instantaneously, was reversible, and was biphasic. The two mercurials inhibited glucose transport as well as glucose oxidation, and increased the mannitol and sucrose spaces of isolated islets. Compared with the effects on insulin release, those on glucose transport and membrane permeability were characterized by a longer latency and/or required higher concentrations of organic mercurial. Apart from a seemingly higher proportion of β-cells exhibiting certain degenerative features, in islets exposed to 0.1mm-chloromercuribenzene-p-sulphonic acid for 60min, no significant differences with respect to β-cell fine structure were noted between non-incubated islets and islets incubated with chloromercuribenzene-p-sulphonic acid or glucose or both. It is suggested that insulin release may be regulated by relatively superficial thiol groups in the β-cell plasma membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Hedeskov C. J., Randle P. J. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970 Jun;118(1):143–154. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode F., Baumann K., Frasch W., Kinne R. Die Bindung von Phlorrhizin an die Bürstensaumfraktion der Rattenniere. Pflugers Arch. 1970;315(1):53–65. doi: 10.1007/BF00587237. [DOI] [PubMed] [Google Scholar]
- Chiba T. [Effect of sulfur-containing compounds on experimental diabetes. V. Effect of thiol and its oxidized type compounds on the liveration of pancreatic insulin]. Yakugaku Zasshi. 1969 Apr;89(4):572–578. doi: 10.1248/yakushi1947.89.4_572. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor of isolated fat cell membranes. J Biol Chem. 1971 Dec 10;246(23):7265–7274. [PubMed] [Google Scholar]
- Douglas W. W., Ishida A., Poisner A. M. The effect of metabolic inhibitors on the release of vasopressin from the isolated neurohypophysis. J Physiol. 1965 Dec;181(4):753–759. doi: 10.1113/jphysiol.1965.sp007795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Determination of free and antibody-bound insulin in insulin treated diabetic patients. Horm Metab Res. 1969 May;1(3):145–146. doi: 10.1055/s-0028-1096835. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Simon E., Täljedal I. B. The pancreatic -cell recognition of insulin secretagogues. I. Transport of mannoheptulose and the dynamics of insulin release. Mol Pharmacol. 1972 Jan;8(1):1–7. [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971 Jul;123(4):513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Evidence for mediated transport of glucose in mammalian pancreatic -cells. Biochim Biophys Acta. 1971 Jul 6;241(1):147–154. doi: 10.1016/0005-2736(71)90312-9. [DOI] [PubMed] [Google Scholar]
- Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965 Oct 8;131(1):541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- Jost K., Rudinger J., Klostermeyer H., Zahn H. Synthese und hypoglycämische Wirkung eines insulinanalogen Cystathionin-Peptides: ein Argument gegen die Beteiligung der intrachenaren Disulfidgruppe bei der Insulinwirkung. Z Naturforsch B. 1968 Aug;23(8):1059–1061. [PubMed] [Google Scholar]
- KEEN H., FIELD J. B., PASTAN I. H. A simple method for in vitro metabolic studies using small volumes of tissue and medium. Metabolism. 1963 Feb;12:143–147. [PubMed] [Google Scholar]
- Kanazawa Y., Orci L., Lambert A. E. Organ culture of fetal rat pancreas. IV. Effects of metabolic inhibitors on insulin release. Endocrinology. 1971 Aug;89(2):576–583. doi: 10.1210/endo-89-2-576. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H. The quantitative histochemistry of the brain; histological sampling. J Histochem Cytochem. 1953 Nov;1(6):420–428. doi: 10.1177/1.6.420. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A. E., Junod A., Stauffahcer W., Jeanrenaud B., Renold A. E. Organ culture of fetal rat pancreas. I. Insulin release induced by caffeine and by sugars and some derivatives. Biochim Biophys Acta. 1969 Sep 2;184(3):529–539. doi: 10.1016/0304-4165(69)90267-0. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Kotler-Brajtburg J., Matschinsky F. M. Kinetics of insulin release from the perfused rat pancreas caused by glucose, glucosamine, and galactose. Proc Natl Acad Sci U S A. 1971 Mar;68(3):536–540. doi: 10.1073/pnas.68.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A., Hellman B. Effect of epinephrine and mannoheptulose on early and late phases of glucose-stimulated insulin release. Metabolism. 1970 Aug;19(8):614–618. doi: 10.1016/0026-0495(70)90018-1. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G., Malaisse-Lagae F. The stimulus-secretion coupling of glucose-induced insulin release. I. Interaction of epinephrine and alkaline earth cations. J Lab Clin Med. 1970 Dec;76(6):895–902. [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Legae F. Effect of thiazides upon insulin secretion in vitro. Arch Int Pharmacodyn Ther. 1968 Jan;171(1):235–239. [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H. Contractile properties of protein threads from sea urchin eggs in relation to cell division. Int Rev Cytol. 1968;23:89–112. doi: 10.1016/s0074-7696(08)60270-3. [DOI] [PubMed] [Google Scholar]
- Schofield J. G. Effect of sulphydryl reagents on the release of ox growth hormone in vitro. Biochim Biophys Acta. 1971 Dec 21;252(3):516–525. doi: 10.1016/0304-4165(71)90154-1. [DOI] [PubMed] [Google Scholar]
- Solberg L. A., Jr, Forte J. G. Differential effects of--SH reagents on transport and electrical properties of gastric mucosa. Am J Physiol. 1971 May;220(5):1404–1412. doi: 10.1152/ajplegacy.1971.220.5.1404. [DOI] [PubMed] [Google Scholar]
- Watkins D., Cooperstein S. J., Lazarow A. Effect of sulfhydryl reagents on permeability of toadfish islet tissue. Am J Physiol. 1970 Aug;219(2):503–509. doi: 10.1152/ajplegacy.1970.219.2.503. [DOI] [PubMed] [Google Scholar]
- Watkins D., Cooperstein S. J., Lazarow A. Effect of sulfhydryl-binding reagents on islet tissue permeability: protection and reversal by thiol compounds. J Pharmacol Exp Ther. 1971 Jan;176(1):42–51. [PubMed] [Google Scholar]



