Abstract
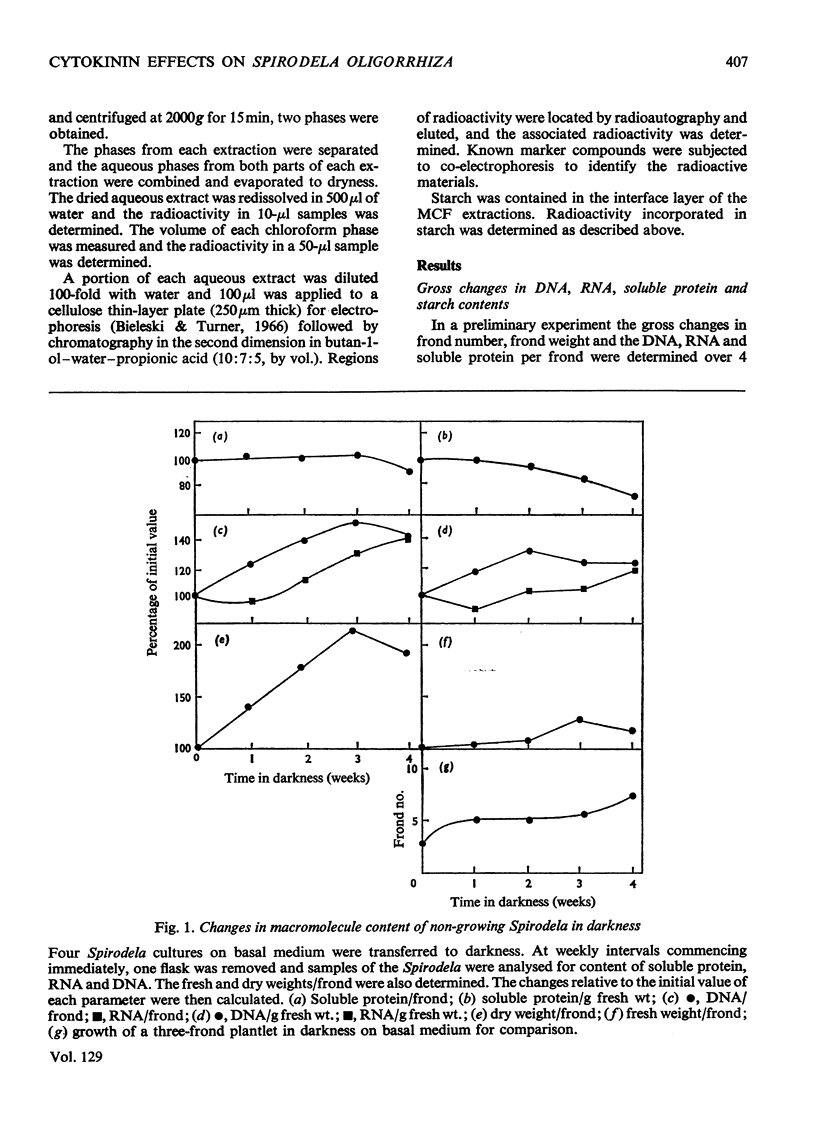
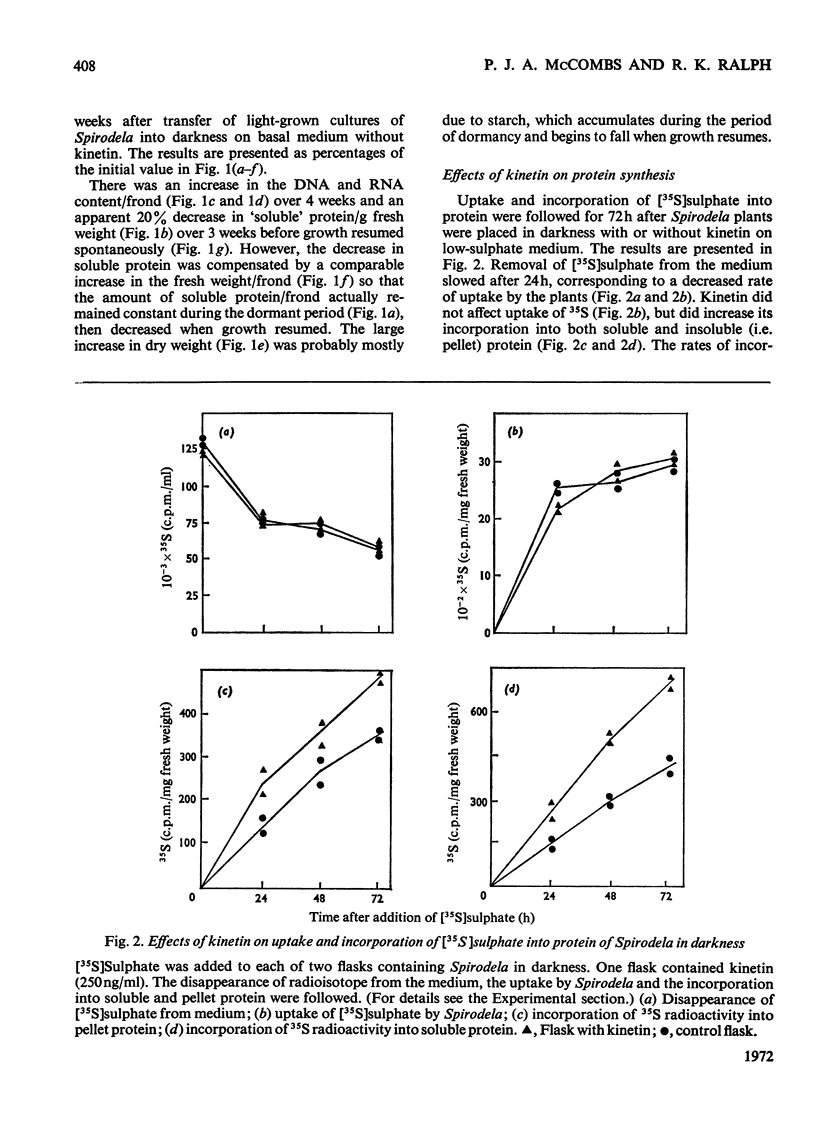
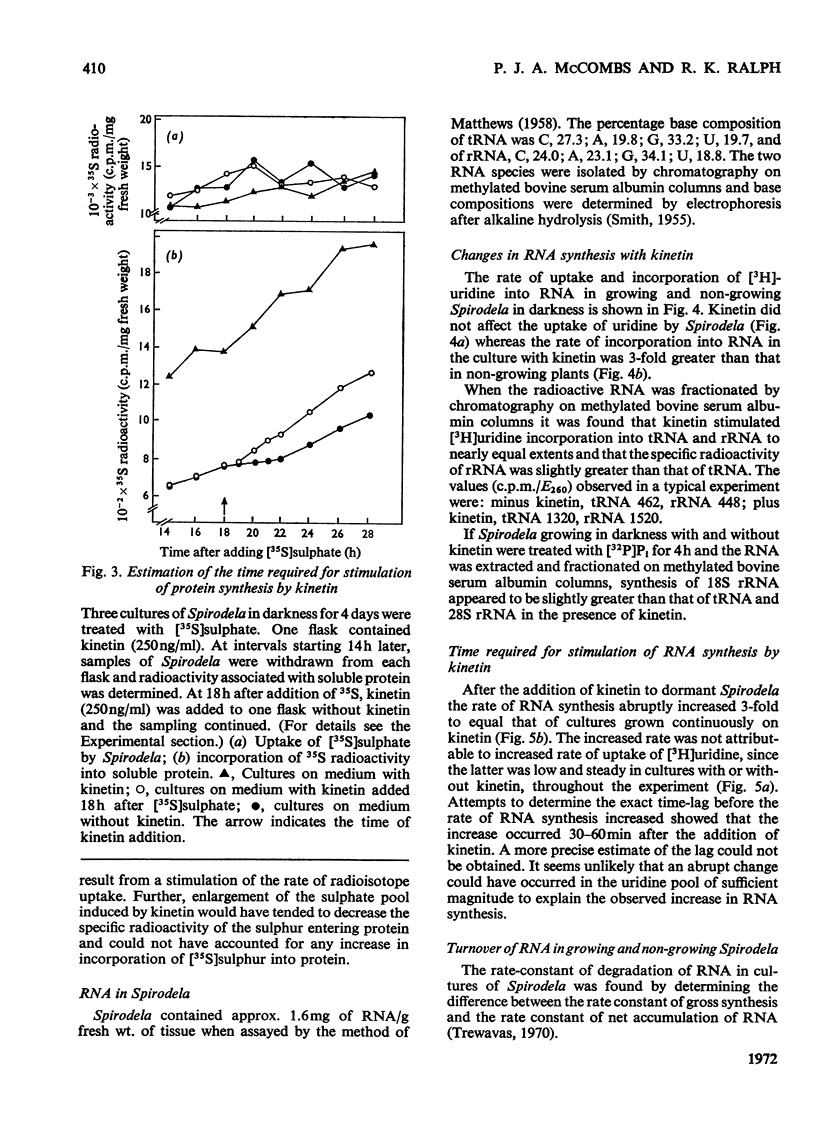
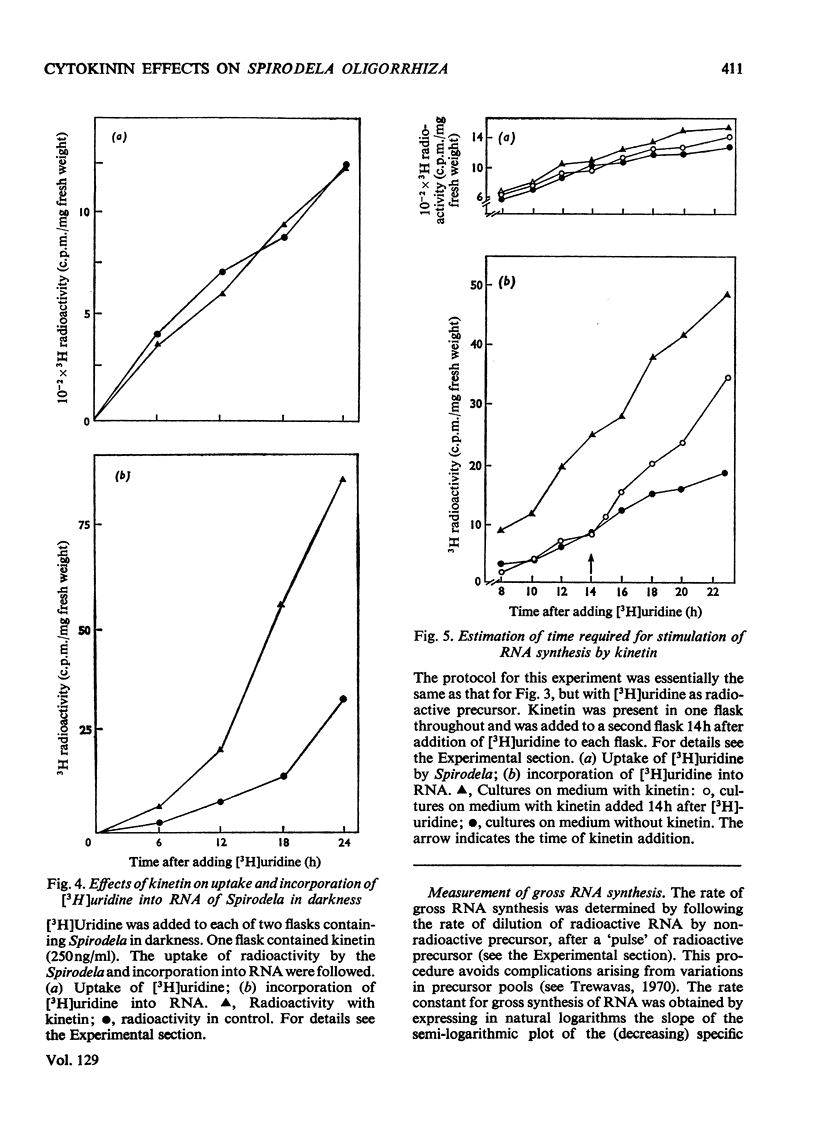
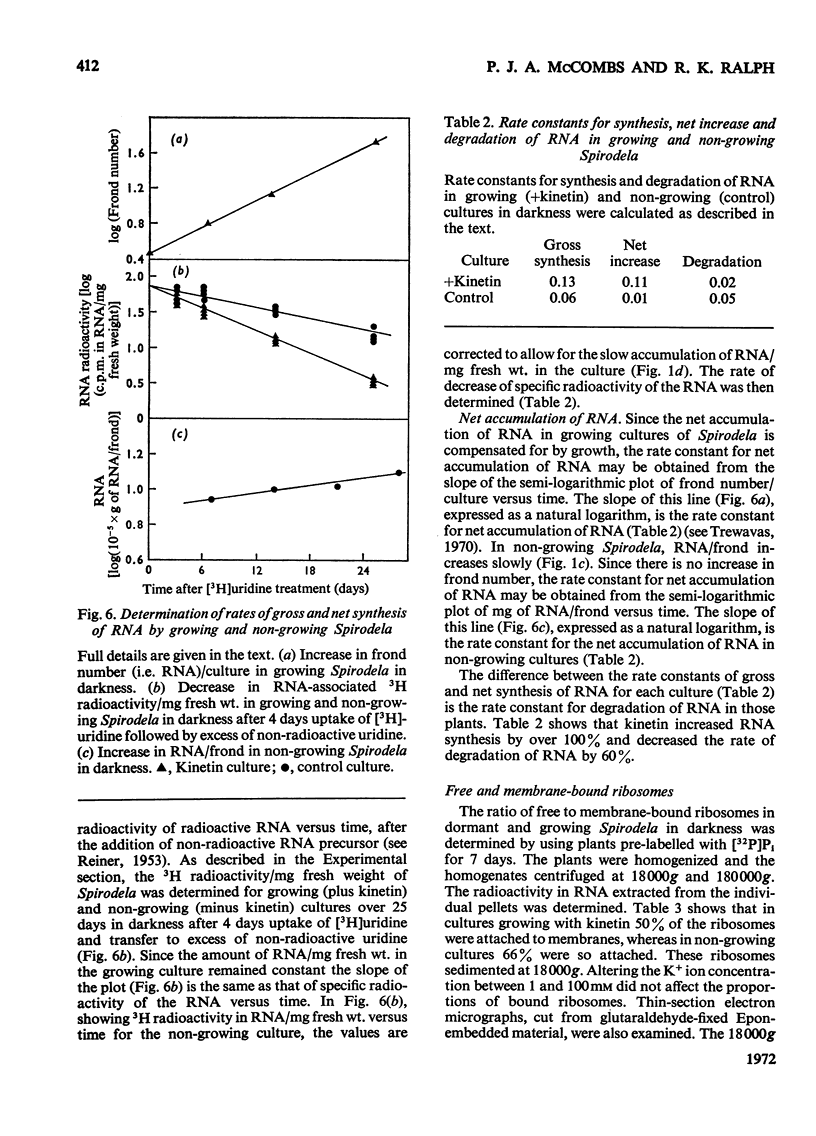
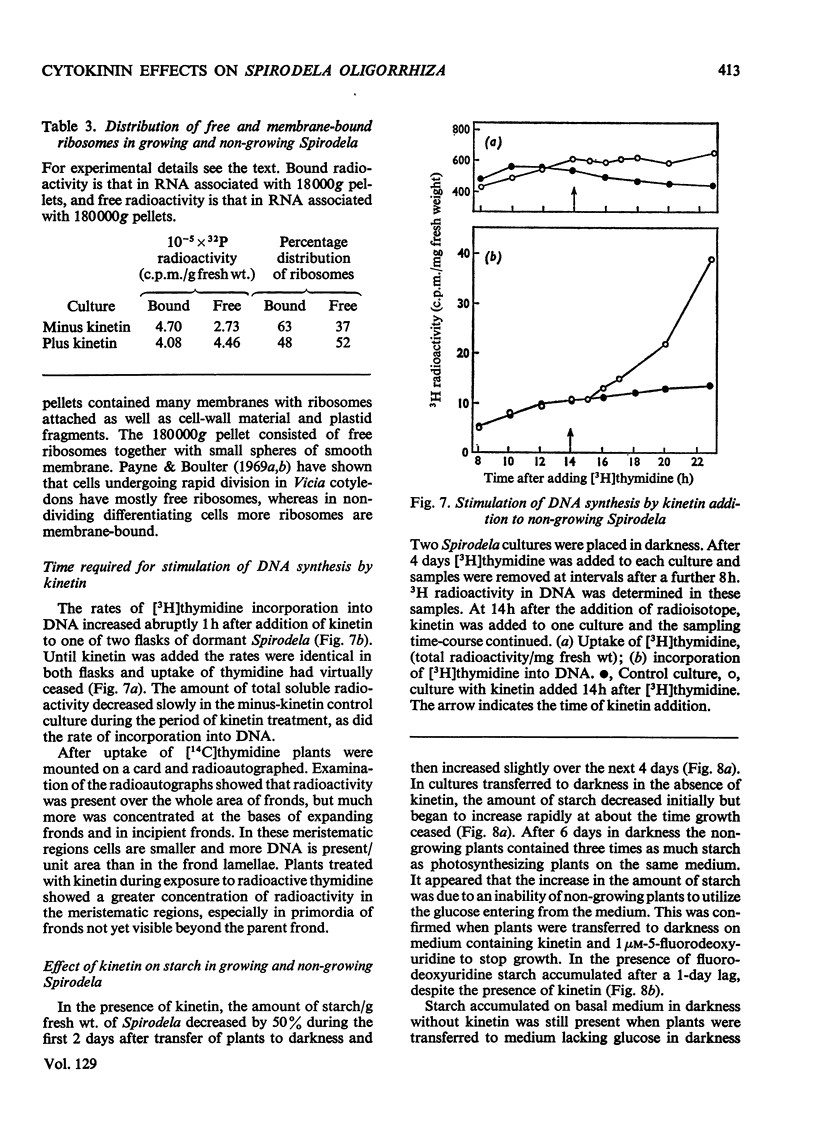
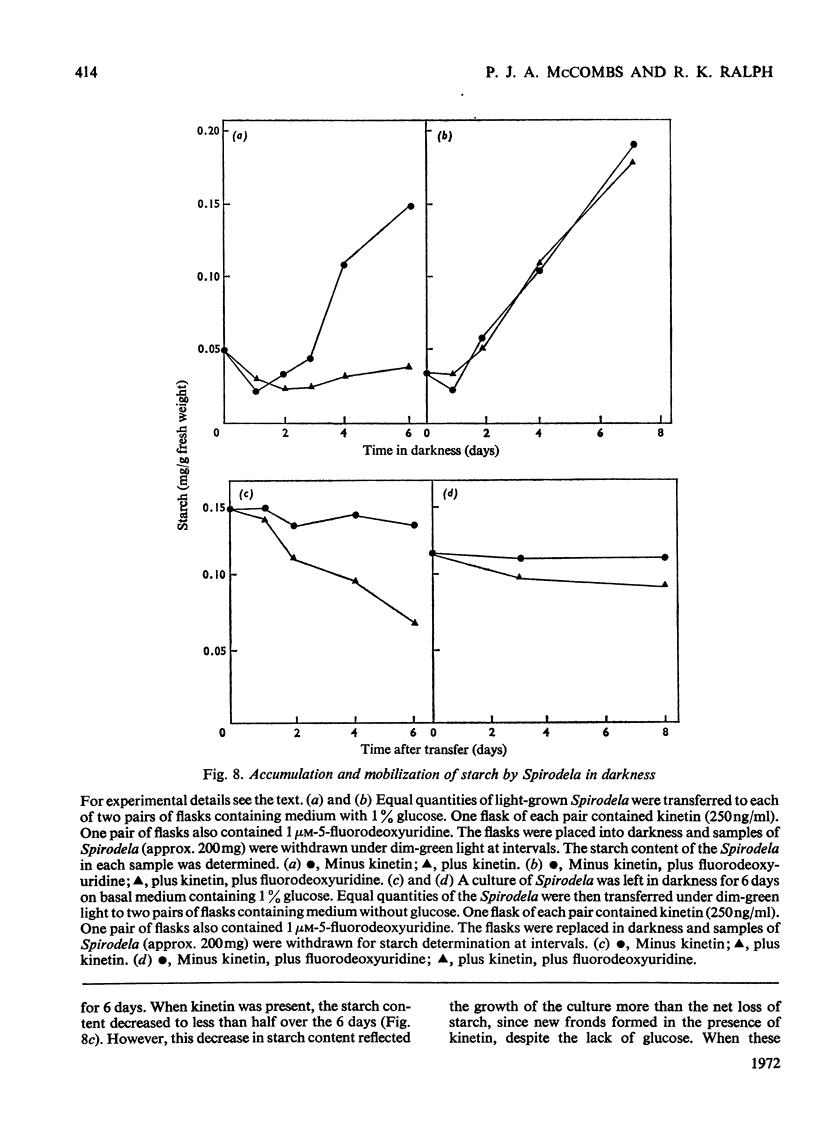
Bacteria-free cultures of Spirodela oligorrhiza continue to increase in frond number for 2 to 3 days after transfer to darkness. There is then no further increase in frond number for 3 to 4 weeks, although DNA, RNA and protein synthesis continue at decreased rates and starch accumulates in the plants. We refer to such `non-growing' plants in darkness as dormant. Adding kinetin to dormant Spirodela initiated increased DNA, RNA and protein synthesis within 1h, although new fronds were not detected until 24h after the addition of kinetin. The frond number then continued to increase. Starch accumulated in dormant plants. Accumulation of starch appeared to be a consequence of inhibition of growth rather than the converse. No evidence was obtained for a block in [14C]glucose metabolism that might explain the lack of growth in darkness in the absence of kinetin. In darkness, more ribosomes were membrane-bound in dormant Spirodela than in Spirodela growing with kinetin. Similarities between the response of Spirodela to darkness, stringent control in bacteria and pleiotypic controls in animal cells are discussed. It is suggested that all three processes are ultimately controlled by specific protein kinases that are individually sensitive to different effectors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Rowan K. S. The effect of 6-furfurylaminopurine on senescence in tobacco-leaf tissue after harvest. Biochem J. 1966 Feb;98(2):401–404. doi: 10.1042/bj0980401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. V., Ralph R. K., Letham D. S. The binding of kinetin to plant ribosomes. Biochem J. 1970 Aug;119(1):75–84. doi: 10.1042/bj1190075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. V., Ralph R. K. Some effects of kinetin on floated Chinese cabbage leaf discs. Biochim Biophys Acta. 1969 May 20;182(1):266–269. doi: 10.1016/0005-2787(69)90547-4. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J., Roy S. C. Growth promoters and the synthesis of protein in plant mitochondria. 1. Effect of kinetin on the incorporation of amino acids into mitochondrial protein. Biochem Biophys Res Commun. 1969 Jun 6;35(5):606–610. doi: 10.1016/0006-291x(69)90447-1. [DOI] [PubMed] [Google Scholar]
- Bieleski R. L., Turner N. A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem. 1966 Nov;17(2):278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Burrows W. J., Skoog F., Leonard N. J. Isolation and identification of cytokinins located in the transfer ribonucleic acid of tobacco callus grown in the presence of 6-benzylaminopurine. Biochemistry. 1971 Jun 8;10(12):2189–2194. doi: 10.1021/bi00788a002. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Collier H. O., Schneider C. Nociceptive response to prostaglandins and analgesic actions of aspirin and morphine. Nat New Biol. 1972 Apr 5;236(66):141–143. doi: 10.1038/newbio236141a0. [DOI] [PubMed] [Google Scholar]
- Cook A. R., Bieleski R. L. Fractionation of plant extracets by thin-layer electrophoretic and chromatographic procedures. Anal Biochem. 1969 Apr 4;28(1):428–435. doi: 10.1016/0003-2697(69)90197-3. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Cocking E. C. Protein synthesis in tomato-fruit locule tissue. Incorporation of amino acids into protein by aseptic cell-free systems. Biochem J. 1967 Jul;104(1):23–33. doi: 10.1042/bj1040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Chen C. Characterization of labeled ribonucleic acid from tissue grown on 14C-containing cytokinins. J Biol Chem. 1967 Oct 10;242(19):4490–4494. [PubMed] [Google Scholar]
- GUTTMAN R. Alterations in nuclear ribonucleic acid metabolism induced by kinetin. J Biophys Biochem Cytol. 1957 Jan 25;3(1):129–132. doi: 10.1083/jcb.3.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Kuo J. F., Miyamoto E. Studies on the mechanism of action of cyclic AMP in nervous and other tissues. Adv Enzyme Regul. 1970;9:113–125. doi: 10.1016/s0065-2571(71)80040-7. [DOI] [PubMed] [Google Scholar]
- Helgeson J. P. The cytokinins. Synthetic and naturally occurring N6-substituted adenine derivatives profoundly affect plant growth. Science. 1968 Sep 6;161(3845):974–981. doi: 10.1126/science.161.3845.974. [DOI] [PubMed] [Google Scholar]
- Hepp K. D., Menahan L. A., Wieland O., Williams R. H. Studies on the action of insulin in isolated adipose tissue cells. II. 3',5'-Nucleotide phosphodiesterase and antilipolysis. Biochim Biophys Acta. 1969 Sep 2;184(3):554–565. doi: 10.1016/0304-4165(69)90269-4. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- JENSEN W. A., POLLOCK E. G., HEALEY P., ASHTON M. KINETIN AND THE NUCLEIC ACID CONTENT OF ONION ROOT TIPS. Exp Cell Res. 1964 Feb;33:523–530. doi: 10.1016/0014-4827(64)90017-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MATTHEWS R. E. Studies on the relation between protein and nucleoprotein particles in turnip yellow mosaic virus infections. Virology. 1958 Apr;5(2):192–205. doi: 10.1016/0042-6822(58)90018-7. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G., Abrams M. A factor mediating interaction of kinins with the genetic material. Biochim Biophys Acta. 1970 Feb 18;199(2):511–518. doi: 10.1016/0005-2787(70)90093-6. [DOI] [PubMed] [Google Scholar]
- Menahan L. A., Wieland O. Interactions of glucagon and insulin on the metabolism of perfused livers from fasted rats. Eur J Biochem. 1969 May 1;9(1):55–62. doi: 10.1111/j.1432-1033.1969.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Osborne D. J. Effect of Kinetin on Protein & Nucleic Acid Metabolism in Xanthium Leaves During Senescence. Plant Physiol. 1962 Sep;37(5):595–602. doi: 10.1104/pp.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINER J. M. The study of metabolic turnover rates by means of isotopic tracers. I. Fundamental relations. Arch Biochem Biophys. 1953 Sep;46(1):53–79. doi: 10.1016/0003-9861(53)90170-2. [DOI] [PubMed] [Google Scholar]
- Rijven A. H., Parkash V. Action of kinetin on cotyledons of fenugreek. Plant Physiol. 1971 Jan;47(1):59–64. doi: 10.1104/pp.47.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft G., Schultz G., Munske K., Hoffmann M. Influence of insulin on cyclic 3',5'-AMP phosphodiesterase activity in liver, skeletal muscle, adipose tissue, and kidney. Diabetologia. 1968 Dec;4(6):322–329. doi: 10.1007/BF01211766. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulson M. Amino acid deprivation of human cells: effects on RNA synthesis, RNA polymerase, and ribonucleoside phosphorylation. Biochim Biophys Acta. 1970 Feb 18;199(2):537–540. doi: 10.1016/0005-2787(70)90100-0. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Hormones and the synthesis and utilization of ribonucleic acids. Prog Nucleic Acid Res Mol Biol. 1966;5:191–250. doi: 10.1016/s0079-6603(08)60235-4. [DOI] [PubMed] [Google Scholar]
- Trewavas A. The Turnover of Nucleic Acids in Lemna minor. Plant Physiol. 1970 Jun;45(6):742–751. doi: 10.1104/pp.45.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


