Abstract
Mice with inactivation of the gene encoding the suppressor of cytokine signaling-1 (SOCS-1) die in neonatal life with an IFN-γ-dependent inflammatory disease dominated by fatty degeneration and necrosis of the liver. To establish the long-term pathological consequences of loss of SOCS-1 in mice, where initial survival was made possible by also deleting the IFN-γ gene, a comparison was made of the lifespan of groups of SOCS-1−/− IFN-γ−/−, SOCS-1+/+ IFN-γ−/− and SOCS-1+/+ IFN-γ+/+ mice. Mice lacking the genes for both SOCS-1 and IFN-γ exhibited an accelerated death rate compared with control groups. Disease states developing selectively in SOCS-1−/− IFN-γ−/− mice were polycystic kidneys, pneumonia, chronic skin ulcers, and chronic granulomas in the gut and various other organs. Mice of all three groups developed cataracts, but disease development was accelerated in the groups lacking IFN-γ. SOCS-1−/− IFN-γ−/− mice exhibited a slightly increased predisposition to the development of T lymphoid leukemia, either spontaneous or radiation-induced. The development of polycystic kidneys may be caused by a developmental defect in renal-tubule organization noted in neonatal SOCS-1−/− mice. The chronic infections and granulomas of SOCS-1−/− IFN-γ−/− mice may be based on autoaggression of SOCS-1−/− T lymphoid and related cells or a functional deficiency of these cells when lacking SOCS-1.
The suppressor of cytokine signaling-1 (SOCS-1) gene, the transcription of which is strongly induced by cytokine stimulation, encodes a cytoplasmic protein that acts in a negative-feedback loop to inhibit signal transduction from activated cytokine receptors (1). The SH2 domain within SOCS-1 interacts with activated, receptor-associated Janus kinase kinases resulting in the inhibition of tyrosine kinase activity (2–4). In addition, the SOCS box at the C terminus of SOCS-1 interacts with the cellular ubiquitination machinery and is thought to target associated signaling molecules for proteasomal degradation (5, 6). It has been shown that, when over-expressed in vitro, SOCS-1 inhibits signals from a diverse range of cytokines, including members of the IL-6 family and thrombopoietin (7), the interferons (7, 8), erythropoietin and interleukin-2 and -3 (2), interleukin-4 (9), growth hormone (10), prolactin (11), insulin-like growth factor I (12), stem-cell factor (13), and tumor necrosis factor 1 (14).
Inactivation of the SOCS-1 gene does not impair fetal development, but SOCS-1−/− mice die during the neonatal period with a disease syndrome dominated by fatty degeneration, necrosis of the liver, and damage to the pancreas, heart, and skin caused by infiltrating T lymphocytes, macrophages, and eosinophils (15–17). This syndrome depends on hyperresponsiveness of SOCS-1−/− cells to stimulation by IFN-γ and the susceptibility of the neonatal mouse liver to the toxic effects of IFN-γ. This fact was clearly established by the prevention of disease in the neonatal period by the administration of antibodies to IFN-γ or by generating mice lacking IFN-γ, in addition to SOCS-1 (18). Interestingly, mice with homozygous inactivation of the SOCS-1 gene that are heterozygous for IFN-γ die prematurely between 3 and 8 weeks of age with a disease state involving polymyositis of all striated muscles, myocarditis, corneal infiltration, and ulcers. In these tissues, the infiltrating cells are again activated T lymphocytes, macrophages, eosinophils, and, less often, neutrophils (19).
The cell type that has been implicated in the possible initiation of both syndromes is the T lymphocyte. These cells may be aberrantly activated either because of hyperresponsiveness to cytokines such as IL-2 or by developing autoresponsiveness either to normal tissue antigens or to surface proteins displayed aberrantly on cells in the absence of SOCS-1 (17).
Young adult mice with homozygous inactivation of the genes encoding both SOCS-1 and IFN-γ seem healthy, although they do have abnormal foci of T and B lymphocytes in their lungs (19). Given the range of different cytokines SOCS-1 can inhibit in vitro, such double knockout mice are of particular interest, because they may represent an in vivo model of multicytokine hyperresponsiveness. The present study was undertaken to determine the long-term consequences of this potentially inadequately restricted cytokine signaling. Because no published account exists of the long-term fate of mice with inactivation of the IFN-γ gene alone, such mice represented an important control group to establish which of the disease states developing in double-knockout mice one might ascribe merely to loss of IFN-γ.
Materials and Methods
Mice.
The generation of mice with homozygous inactivation of the SOCS-1 gene has been described (15). Homozygous IFN-γ−/− mice were obtained from The Jackson Laboratory; by interbreeding SOCS-1+/− mice with IFN-γ+/− mice, progeny of the three genotypes were generated for study—SOCS-1−/− IFN-γ−/−, SOCS-1+/+ IFN-γ−/−, and SOCS-1+/+ IFN-γ+/+. Mice were genotyped by Southern blot analysis of genomic DNA from tail tips, as described (18). All mice were of mixed genetic background of C57BL/6 and 129/Sv. The study mice were housed in protected animal quarters that were monitored regularly for the presence of pathogenic viruses and bacteria. Mice of the study groups were clinically inspected daily for the lifespan of the SOCS-1−/− IFN-γ−/− mice.
Analysis of Mice.
Mice were killed when clinically ill, and the brain, thymus, thyroid, heart, lung, salivary glands, sternum, femur, tibia, skin, liver, spleen, pancreas, kidneys, small bowel, bladder, skeletal muscle, and uterus or testes were fixed in 10% buffered formalin; the tissues were blocked, and sections were stained routinely with hematoxylin and eosin. When leukemias developed, these tissues were subjected to FACS analysis as described (19). In addition to the study groups, aging mice were analyzed at intervals by performing cell counts on orbital blood; also, absolute and differential cell counts were performed on peritoneal, spleen, and marrow populations and were subjected to histological examination.
Irradiation.
To induce lymphoid leukemia, mice aged 28 to 31 days were given an initial dose of 168 cGy of whole-body γ-irradiation from a 137Cs source followed by two further doses of 168 cGy at weekly intervals.
Statistical Analysis.
Comparison of survival curves from irradiated mice was performed by logrank test by using PRISM VERSION 3.0 statistical software (GraphPad, San Diego). Other statistical analyses used the Student's t test.
Results
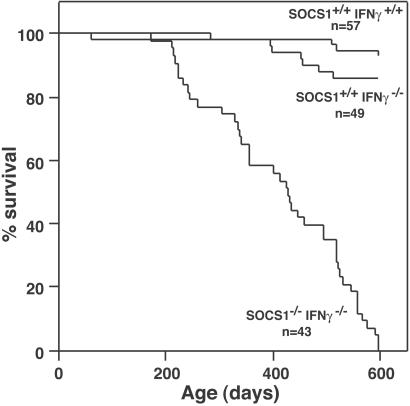
Groups of 43–57 contemporaneous male and female SOCS-1−/− IFN-γ−/− mice and control SOCS-1+/+ IFN-γ−/− and SOCS-1+/+ IFN-γ+/+ mice were monitored to determine their lifespans and the disease states present when moribund. As shown in Fig. 1, SOCS-1−/− IFN-γ−/− mice exhibited an accelerated death rate, and all had become moribund by 600 days of age. In contrast, most control SOCS-1+/+ IFN-γ−/− and SOCS-1+/+ IFN-γ+/+ mice were still in apparent good health at this age. To terminate this two-year study after the death of the last experimental animal, all surviving control mice were killed when aged 575–753 days, and these mice then were analyzed histologically.
Figure 1.
Survival curves of combined male and female SOCS-1−/− IFN-γ−/−, SOCS-1+/+ IFN-γ−/−, and SOCS-1+/+ IFN-γ+/+ mice. n = number of mice surveyed in each group.
Because no long-term study has been reported on the consequences of deletion of the IFN-γ gene, 12 mice of both control groups also were analyzed for their hematological status when aged more than 600 days. The only abnormalities observed in these control groups were an elevated number of lymphocytes in the peripheral blood of SOCS-1+/+ IFN-γ−/− mice (12,370 ± 8,020 μl−1 in SOCS-1+/+ IFN-γ−/− mice vs. 6,320 ± 4,670 μl−1 in SOCS-1+/+ IFN-γ+/+ mice; P = 0.05), a variable enlargement of the spleen in SOCS-1+/+ IFN-γ−/− mice (230 ± 402 mg vs. 127 ± 61 mg in control SOCS-1+/+ IFN-γ+/+ mice; P = 0.43), and a marked elevation of peritoneal cell numbers in both groups, lymphocytes being mainly responsible for the increased cell numbers (total lymphocyte numbers for SOCS-1+/+ IFN-γ−/− mice, 74.4 ± 73.3 × 106; and for SOCS-1+/+ IFN-γ+/+ mice, 34.5 ± 23.9 × 106 vs. 3 ± 2 × 106 for normal young adult mice; P < 0.01). All other parameters in the blood, spleen, and marrow populations were within the normal limits for normal young adult mice.
Table 1 lists the frequency of various pathological states observed in the three groups under study. Several disease states were noted in SOCS-1−/− IFN-γ−/− mice that were either not present, or at significantly lower frequency, in control mice. These disease states were enlarged polycystic kidneys, pneumonia, chronic ulceration of the skin of the tail, flank regions, or ears (regions accessible to scratching), granulomas with infiltrating lymphocytes, macrophages and eosinophils in the gut, and a miscellany of chronic granulomas located in the abdomen or uterus and involving the same cell types.
Table 1.
Pathology of SOCS-1−/− IFNγ−/− mice and controls
| Parameter | Frequency, %
|
||
|---|---|---|---|
| SOCS-1−/− IFNγ−/− (n = 37) | SOCS-1+/+ IFNγ−/− (n = 44) | SOCS-1+/+ IFNγ+/+ (n = 55) | |
| Polycystic kidney | 46 | 2 | 0 |
| Lung–pneumonia | 59 | 5 | 2 |
| –adenoma | 3 | 9 | 11 |
| Chronic skin ulcers | 35 | 7 | 0 |
| Chronic abscess, miscellaneous site | 22 | 2 | 0 |
| Gut–granuloma | 38 | 0 | 2 |
| Cardiac infarct/infiltration | 8 | 0 | 0 |
| Lymphoid leukemia | 11 | 5 | 7 |
| Reticulum cell sarcoma | 5 | 7 | 22 |
| Cataracts | 81 | 98 | 75 |
| Lymphoid foci | |||
| –liver | 32 | 16 | 67 |
| –lung | 86 | 34 | 73 |
| –kidney | 65 | 25 | 67 |
| –bladder | 46 | 11 | 25 |
| –salivary glands | 29 | 25 | 87 |
| –pancreas | 6 | 7 | 71 |
| Fatty proximal renal tubules | 14 | 30 | 7 |
| Marrow G > M | 84 | 66 | 76 |
Data for SOCS-1+/+ IFNγ−/− mice (with five exceptions) and SOCS-1+/+ IFNγ+/+ mice (with one exception) are from remaining survey mice killed when apparently healthy at 575–753 days of age after all SOCS-1−/− IFNγ−/− mice had died. Marrow G > M indicates an excess of granulocytic cells in the marrow vs. mononuclear lymphoid and erythroid cells.
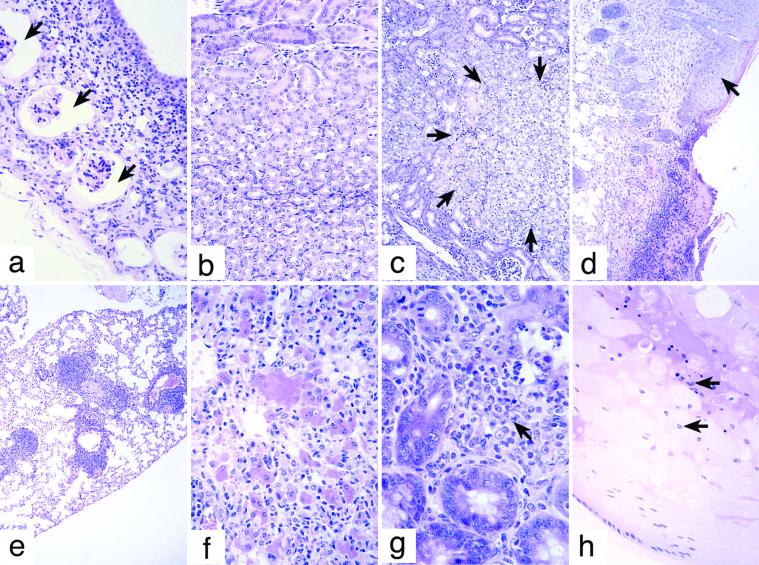
The development of polycystic kidneys seemed to require loss of the SOCS-1 gene. Typically, only one kidney was involved, and the walls of the cystic spaces contained some surviving glomeruli of normal appearance but dilated Bowman's capsules and with infiltration by lymphocytes, macrophages, eosinophils, and plasma cells (Fig. 2a). The lesion was of interest because neonatal SOCS-1−/− mice exhibit a derangement or delay in establishing a normal architectural pattern of renal medullary collecting tubules (Fig. 2 b and c).
Figure 2.
(a) Cyst wall of a polycystic kidney in a SOCS-1−/− IFN-γ−/− mouse showing dilated Bowmans capsules around surviving glomeruli (arrows) and a lymphocyte and plasma-cell infiltrate in the wall. (b) Two-week-old SOCS-1+/+ kidney showing a normal pattern of medullary tubules. (c) Two-week-old SOCS-1−/− kidney showing disorganization of collecting tubule development in the medulla region (indicated by arrows). (d) Edge of a chronic skin ulcer in a SOCS-1−/− IFN-γ−/− mouse. Note the hyperplastic epithelium at the edge of the ulcer (arrow). (e) Lymphoid foci in the lung of a SOCS-1−/− IFN-γ−/− mouse. (f) Pneumonia in a SOCS-1−/− IFN-γ−/− mouse with characteristic enlarged eosinophilic cells, infiltrating lymphocytes, macrophages, and neutrophils. (g) Intestinal wall of a SOCS-1−/− IFN-γ−/− mouse showing granuloma tissue (arrow) at the base of the villi. (h) Cataract in the lens of a SOCS-1−/− IFN-γ−/− mouse with aberrant nuclei of equatorial lens cells (arrows) and degeneration of the lens matrix.
The pneumonia developed by SOCS-1−/− IFN-γ−/− mice (Fig. 2f) involved patchy areas of the lung with a chronic infiltrate of lymphoid cells, macrophages, and eosinophils, with a few neutrophils. A characteristic feature of these lesions was the presence of enlarged eosinophilic cells, either alveolar macrophages or alveolar cells, often with large eosinophilic crystals in the cytoplasm or lying free between the cells.
The chronic skin ulcers (Fig. 2d) and various granulomatous lesions in the skin, gut (Fig. 2g), and abdominal organs also involved populations of lymphocytes, macrophages, eosinophils, and less often neutrophils; these states seemed to have been chronic in nature.
Disease states observed in lower frequency in SOCS-1−/− IFN-γ−/− mice than in control mice included adenomas of the lung and reticulum cell sarcomas. Both diseases characteristically develop in mice later in life. Therefore, the difference is likely to be based on the significantly older average age of the control mice when analyzed (634 ± 26 days for SOCS-1+/+ IFN-γ−/− mice and 655 ± 42 days for SOCS-1+/+ IFN-γ+/+ mice) vs. the average age of the experimental SOCS-1−/− IFN-γ−/− mice at death (411 ± 192 days).
Cataract formation, which was usually bilateral, was observed in a surprisingly high frequency in all three groups of mice. Cataracts commenced with aberrant dispersion of Feulgen-positive degenerating equatorial lens nuclei into the more central regions of the lens, followed by breakdown of the homogeneous lens protein, often with vacuole formation (Fig. 2h). No extraneous infiltrating cells were present in any of the affected lenses. From the data in Table 1, cataract formation was not linked obviously with loss either of the SOCS-1 or IFN-γ genes. However, at autopsy, the disease was less advanced in SOCS-1+/+ IFN-γ+/+ mice than in the other two groups. In support of this difference, a clinical analysis of the eyes of mice of all three groups undertaken when the mice were aged 283–510 days revealed frequencies of clinically obvious cataracts of 72% for SOCS-1−/− IFN-γ−/− mice, 73% for SOCS-1+/+ IFN-γ−/− mice, and only 3% for SOCS-1+/+ IFN-γ+/+ mice, also suggesting that loss of the IFN-γ gene had accelerated the development of this disease.
Mice of all three groups exhibited a high frequency of lymphoid foci present in the liver, lung (Fig. 2e), kidney, bladder, salivary glands, and pancreas. This finding paralleled the elevated levels of lymphocytes in the peripheral blood and peritoneal cavity. The lymphoid foci were not more extensive in SOCS-1−/− IFN-γ−/− mice than in control mice. They were tightly circumscribed in nature and contained both T and B lymphoid cells with no germinal centers, and exhibited little or no mitotic activity. Adjacent tissue was not damaged, and the initiating cause of these foci was not established. Focal accumulations of lymphoid cells in the lungs of young adult SOCS-1−/− IFN-γ−/−, at which age the lesions seemed to be related to loss of SOCS-1, have been noted in previous studies (19). A survey of small groups of mice of all three genotypes at the age of 360 days failed to reveal lymphoid foci in other organs, and the development of these foci seemed to be progressive with advancing age in all three genotypes.
None of the SOCS-1−/− IFN-γ−/− mice developed the fatty degeneration and necrosis of the liver characteristic of young SOCS-1−/− mice (15). The presence of lipid in hepatocytes and proximal renal-tubule cells was noted in some of the very old mice of all three groups; it seemed to be a nonspecific change with increasing age, as was the presence of an elevated population of granulocytic cells in the bone marrow.
Lymphoid Leukemia.
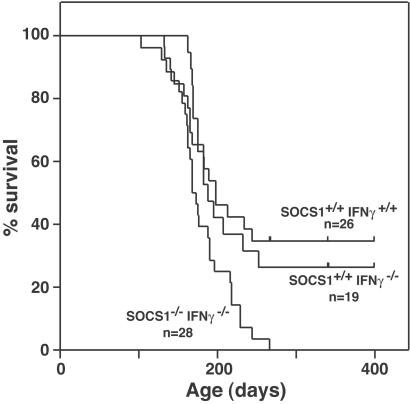
The development of four cases of lymphoid leukemia in the SOCS-1−/− IFN-γ−/− mice at an earlier age (mean age = 225 days) than cases developing in control mice (for SOCS-1+/+ IFN-γ−/− mice, mean age = 573 days; for SOCS-1+/+ IFN-γ+/+ mice, mean age = 608 days) was intriguing in view of the activated state of T lymphocytes noted in young adult SOCS-1−/− IFN-γ−/− mice (19). However, no further cases of lymphoid leukemia developed. It was possible that the activated state of T lymphocytes might render them more susceptible to leukemic transformation than the cells of control mice. To further explore this possibility, additional groups of young mice of all three genotypes were subjected to a leukemogenic regimen of whole-body irradiation (three 168 cGy whole-body irradiations). As shown in Fig. 3, irradiation induced a high frequency of lymphoid leukemia in mice of all three genotypes, with leukemia developing at a moderately but significantly higher frequency, but no earlier, in SOCS-1−/− IFN-γ−/− mice than in control mice. Fluorescence activated cell sorter analysis of 23 of the leukemias in the three groups showed that all were T lymphoid (CD4+CD8+) with the cells exhibiting a varying elevated level of expression of the activation marker CD44 (data not shown). All leukemic mice exhibited the typical pathology of lymphoid leukemia involving thymus, lymph nodes, liver, kidney, and spleen. In view of the frequent infiltration of T lymphocytes in the skin, pancreas, skeletal muscle, heart, and cornea of sick neonatal and young adult SOCS-1−/− mice (15, 19), it was curious that leukemic-cell infiltrates in irradiated SOCS-1−/− IFN-γ−/− mice were rare or not present in these tissues.
Figure 3.
Survival of SOCS-1−/− IFN-γ−/−, SOCS-1+/+ IFN-γ−/−, and SOCS-1+/+ IFN-γ+/+ mice after three 168 cGy whole-body irradiations. All dying mice had histologically verified lymphoid leukemia. n = number of mice in each group, with vertical bars denoting the ages of living mice in the SOCS-1+/+ IFN-γ−/− and SOCS-1+/+ IFN-γ+/+ groups at the termination of the experiment. The survival curve for SOCS-1−/− IFN-γ−/− mice is significantly different from that of SOCS-1+/+ IFN-γ−/− and SOCS-1+/+ IFN-γ+/+ mice; P = 0.007.
Discussion
SOCS-1 can attenuate signaling initiated by many cytokines. Mice lacking SOCS-1 die in neonatal life with liver degeneration and inflammatory lesions in multiple tissues, but disease development also can be prevented by deleting the IFN-γ gene (18). This finding has highlighted the particular importance of IFN-γ for disease development in young mice lacking SOCS-1. Although SOCS-1−/− IFN-γ−/− mice seem healthy as young adults, they are in a potentially abnormal state because, based on the in vitro promiscuity of SOCS-1 action, the absence of SOCS-1 in these mice might allow excessive responses to multiple cytokines. The SOCS-1 protein possibly may have additional intracellular actions whose importance may emerge later in life. Therefore, it was of interest to follow the fate of SOCS-1−/− mice whose survival had been made possible by the additional deletion of the IFN-γ gene.
The clearest outcome of the present long-term study of SOCS-1−/− IFN-γ−/− mice was the failure of the mice at any time in their lives to develop hepatocyte damage as seen in neonatal SOCS-1−/− mice (15–17) or the polymyositis or corneal inflammation developing in young adult SOCS-1−/− IFN-γ+/− mice (19). This result indicates that IFN-γ is absolutely necessary for the development of these lesions in SOCS-1−/− mice and is not merely an agent that accelerates the onset of these diseases.
However, although neonatal or early adult disease was avoided by removing IFN-γ, loss of SOCS-1 significantly shortened the lifespan of the mice. The major causes of premature death were the development of polycystic kidneys and a group of infections or inflammatory states in which chronic pneumonia predominated. Commonly, more than one disease state was present in individual mice.
The experimental design involved the killing and examination of control mice remaining alive after the death of the last experimental mouse. This procedure was suboptimal, because it was likely to have artificially reduced the frequency of certain diseases, such as reticulum cell sarcoma or lung adenomas, in the control group. However, these diseases were noted only in control mice aged 520–742 days and, because few SOCS-1−/− IFN-γ−/− mice reached such an age (Fig. 1), they cannot rationally be regarded as having avoided these diseases because of the absence of SOCS-1.
The high frequency of polycystic kidneys was restricted to SOCS-1−/− IFN-γ−/− mice, which suggests that one can ascribe this lesion to the loss of the SOCS-1 protein. In this context, it is of interest that neonatal SOCS-1−/− mice exhibit a consistent deficiency, or delay, in the organization of renal medullary tubules into a regular pattern (Fig. 2c). It is conceivable that this developmental abnormality may lead to failure of occasional medullary tubules to make adequate connections with cortical proximal tubules, leading to tubular dilatation and cyst formation by transfiltrates from the glomeruli involved. Most such polycystic kidneys had infiltrates of lymphocytes and plasma cells in the cyst wall and sometimes chronic granulomas in adjacent tissues, but these may have been secondary to cyst formation.
The occurrence of some early T lymphoid leukemias in the SOCS-1−/− IFN-γ−/− mice was of interest because there are aberrant ratios of T lymphocyte subsets and activation particularly of CD8+ cells in mice lacking SOCS-1 (15–17, 19). The activated state of T lymphocytes in the absence of SOCS-1 might possibly have rendered them more susceptible to neoplastic transformation. However, whereas whole-body irradiation was somewhat more effective in inducing T lymphoid tumors in SOCS-1−/− IFN-γ−/− than in control mice, the differences were relatively minor.
The T lymphocytes or natural killer T lymphocytes of SOCS-1−/− mice have been shown to be autoaggressive (20), and transplantation of SOCS-1−/− bone-marrow cells to irradiated syngeneic recipients results in premature death (ref. 17 and unpublished data). Therefore, the present chronic inflammatory disease of the lungs, skin, and bowel in aging SOCS-1−/− IFN-γ−/− mice might have been based on autoaggression of T lymphoid and related cells. However, the lesions did not resemble those of graft-versus-host disease (21, 22) or autoimmune diseases. Of the inflammatory diseases, the most complex were the pneumonia lesions involving enlargement of alveolar cells, infiltration by macrophages, neutrophils, and eosinophils, and the development of large, Charcot–Leyden-like crystals, often within the lumen of bronchi. There were no obvious foci of bacteria or fungi in any of the inflammatory lesions, but the presence of microbial infections cannot be excluded.
If the inflammatory lesions were based on microbial infections, this would suggest an inadequate capacity of SOCS-1−/− IFN-γ−/− mice to eliminate microorganisms of possibly low pathogenicity. It has been documented that lack of IFN-γ results in increased susceptibility to a number of infections (23, 24) and might possibly contribute to the development of chronic infections. However, such lesions occurred only, or earlier, in SOCS-1−/− IFN-γ−/− mice, and, therefore, the defect, if real, seems to be ascribable mainly to lack of SOCS-1. The lymphoid foci developing in SOCS-1−/− IFN-γ−/− mice seemed unrelated to the chronic inflammatory lesions and were equally frequent and extensive in the control mice.
The development of cataracts in a high proportion of SOCS-1−/− IFN-γ−/− mice, with no evidence of cellular infiltration in the eye, was unexpected, but the disease also developed with similar frequency in both control groups. The disease may have been dependent on the particular genotype of the mice used in the present study, but these genotypes are commonly used strains. Although the opacity of the eyes was quite evident on clinical inspection and commenced under the age of 6 months, its occurrence may have been missed by earlier investigators. Numerous mutations have been described as predisposing to cataract formation (25), but the disease has not been noted as a consequence of loss of IFN-γ. However, the lesions were less advanced in SOCS-1+/+ IFN-γ+/+ mice, and clinical examination indicated that cataract formation developed earlier in both groups lacking IFN-γ. Therefore, the data suggest that loss of IFN-γ can accelerate cataract formation.
It is worthy of comment that aging SOCS-1−/− IFN-γ−/− mice failed to develop the various pathological changes noted as following upon overexpression of IL-6 (26), GM-CSF (27), or stem cell factor (28). This finding suggests that the observed ability of SOCS-1, when over-expressed, to inhibit signaling by these agents in vitro may be misleading because no evidence of sustained overstimulation of cells by these regulators was observed when SOCS-1 was missing in vivo.
Loss of the IFN-γ gene alone had little impact on the lifespan or pattern of disease development in the mice surveyed, other than the possible acceleration of cataract development and a lower frequency of lymphoid foci in some organs. The low frequency of obvious infections in mice lacking IFN-γ was surprising, but one may ascribe it to the protected animal rooms in which the study groups were housed. It has been reported that mice with inactivation of the IFN-γ receptor α-chain gene show an increased susceptibility to carcinogen-induced tumor formation (29, 30), but enhanced tumor development was not observed in either of the present groups of mice lacking IFN-γ.
The overall results confirm and extend earlier data indicating that most disease development after the loss of SOCS-1 requires the presence of IFN-γ (18, 19). The only disease states developing in mice lacking both SOCS-1 and IFN-γ were polycystic kidneys and a miscellany of chronic infections or inflammatory lesions, and, therefore, these represented the major types of late-developing IFN-γ-independent disease one may ascribe to the loss of SOCS-1.
Acknowledgments
We thank Janelle Mighall and Sally Cane for technical assistance, Steven Mihajlovic and Ellen Tsui for histology, and Katya Gray for animal husbandry. This work was supported by the National Health and Medical Research Council, Canberra, the Anti-Cancer Council of Victoria, an Australian Government Cooperative Research Centres Program Grant, National Institutes of Health Grant CA22556, the J. D. and L. Harris Trust, and Australian Medical Research and Development (AMRAD) Corp., Melbourne. Commercial rights to SOCS-1 have been assigned to AMRAD Corporation, who also funded part of this work.
Abbreviation
- SOCS-1
suppressor of cytokine signaling-1
References
- 1.Krebs D L, Hilton D J. J Cell Sci. 2000;113:2813–2819. doi: 10.1242/jcs.113.16.2813. [DOI] [PubMed] [Google Scholar]
- 2.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al. Nature (London) 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 3.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et al. Nature (London) 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson S E, Willson T A, Farley A, Starr R, Zhang J G, Baca M, Alexander W S, Metcalf D, Hilton D J, Nicola N A. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamura T, Sato S, Haque D, Liu L, Kaelin W G, Jr, Conaway R C, Conaway J W. Genes Dev. 1998;12:3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J G, Farley A, Nicholson S E, Willson T A, Zugaro L M, Simpson R J, Moritz R L, Cary D, Richardson R, Hausmann G, et al. Proc Natl Acad Sci USA. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. Nature (London) 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 8.Song M M, Shuai K. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 9.Losman J A, Chen X P, Hilton D, Rothman P. J Immunol. 1999;162:3770–3774. [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen J A, Lindberg K, Hilton D J, Nielsen J H, Billestrup N. Mol Endocrinol. 1999;13:1832–1843. doi: 10.1210/mend.13.11.0368. [DOI] [PubMed] [Google Scholar]
- 11.Pezet A, Favre H, Kelly P A, Edery M. J Biol Chem. 1999;274:24497–24502. doi: 10.1074/jbc.274.35.24497. [DOI] [PubMed] [Google Scholar]
- 12.Zong C S, Chan J, Levy D E, Horvath C, Sadowski H B, Wang L H. J Biol Chem. 2000;275:15099–15105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]
- 13.De Sepulveda P, Okkenhaug K, Rose J L, Hawley R G, Dubreuil P, Rottapel R. EMBO J. 1999;18:904–915. doi: 10.1093/emboj/18.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita Y, Naka T, Kawazoe Y, Fujimoto M, Narazaki M, Nakagawa R, Fukuyama H, Nagata S, Kishimoto T. Proc Natl Acad Sci USA. 2000;97:5405–5410. doi: 10.1073/pnas.090084797. . (First Published May 2, 2000; 10.1073/pnas.090084797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starr R, Metcalf D, Elefanty A G, Brysha M, Willson T A, Nicola N A, Hilton D J, Alexander W S. Proc Natl Acad Sci USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Proc Natl Acad Sci USA. 1998;95:15577–15582. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marine J C, Topham D J, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle J N. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 18.Alexander W S, Starr R, Fenner J E, Scott C L, Handman E, Sprigg N S, Corbin J E, Cornish A L, Darwiche R, Owczarek C M, et al. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 19.Metcalf D, Di Rago L, Mifsud S, Hartley L, Alexander W S. Proc Natl Acad Sci USA. 2000;97:9174–9179. doi: 10.1073/pnas.160255197. . (First Published July 25, 2000; 10.1073/pnas.160255197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naka T, Tsutsui H, Fujimoto M, Kawazoe Y, Kohzaki H, Morita Y, Nakagawa R, Narazaki M, Adachi K, Yoshimoto T, et al. Immunity. 2001;14:535–545. doi: 10.1016/s1074-7613(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 21.Rappaport H, Khalil A, Halle-Pannenko O, Pritchard L, Dantchev D, Mathe G. Am J Pathol. 1979;96:121–142. [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries M J, Vos O. J Natl Cancer Inst. 1959;23:1403–1439. [Google Scholar]
- 23.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 24.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 25.Francis P J, Berry V, Moore A T, Bhattacharya S. Trends Genet. 1999;15:191–196. doi: 10.1016/s0168-9525(99)01738-2. [DOI] [PubMed] [Google Scholar]
- 26.Fattori E, Della Rocca C, Costa P, Giorgio M, Dente B, Pozzi L, Ciliberto G. Blood. 1994;83:2570–2579. [PubMed] [Google Scholar]
- 27.Lang R A, Metcalf D, Cuthbertson R A, Lyons I, Stanley E, Kelso A, Kannourakis G, Williamson D J, Klintworth G K, Gonda T J, et al. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- 28.Kunisada T, Lu S Z, Yoshida H, Nishikawa S, Mizoguchi M, Hayashi S, Tyrrell L, Williams D A, Wang X, Longley B J. J Exp Med. 1998;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan D H, Shankaran V, Dighe A S, Stockert E, Aguet M, Old L J, Schreiber R D. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankaran V, Ikeda H, Bruce A T, White J M, Swanson P E, Old L J, Schreiber R D. Nature (London) 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]