Abstract
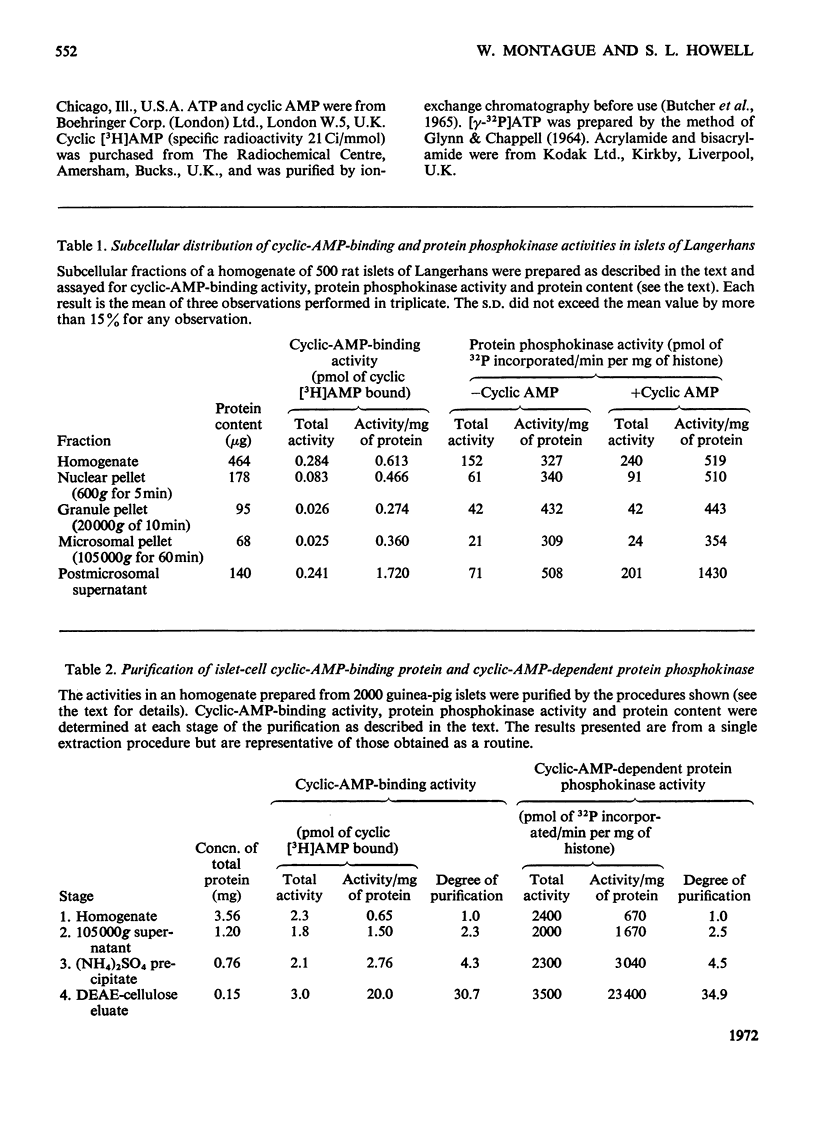
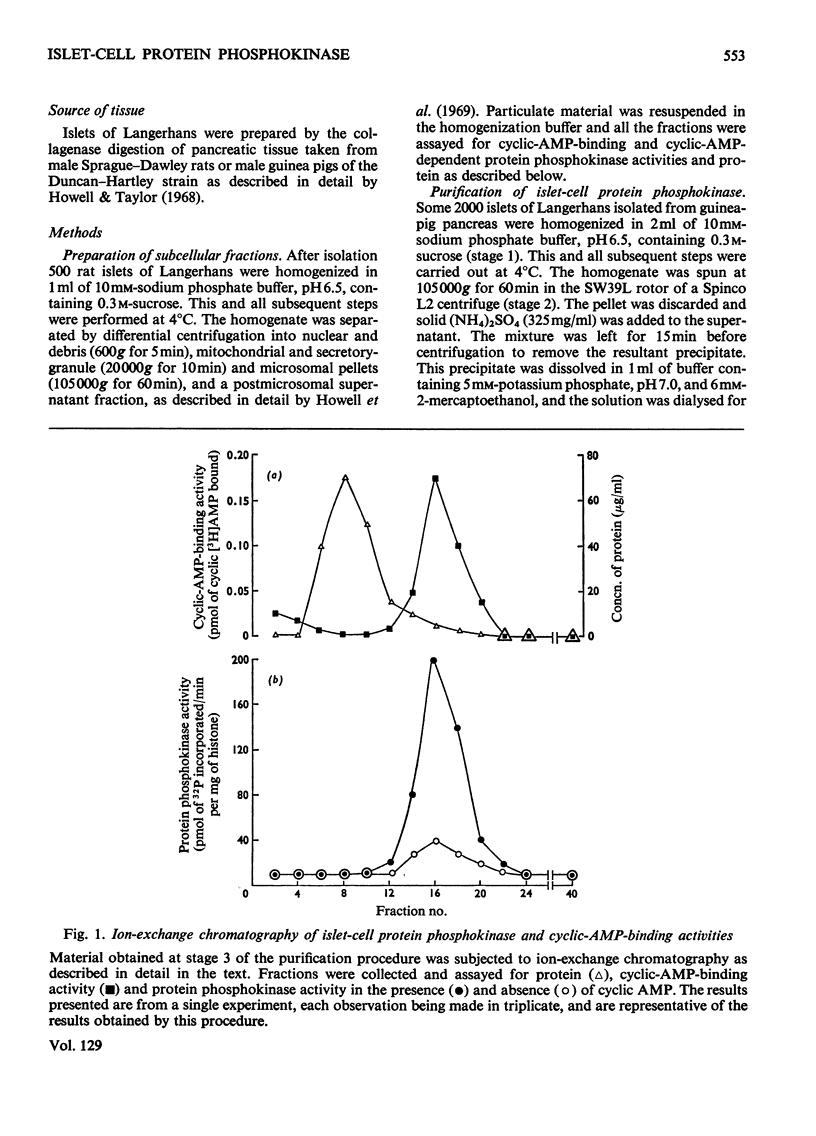
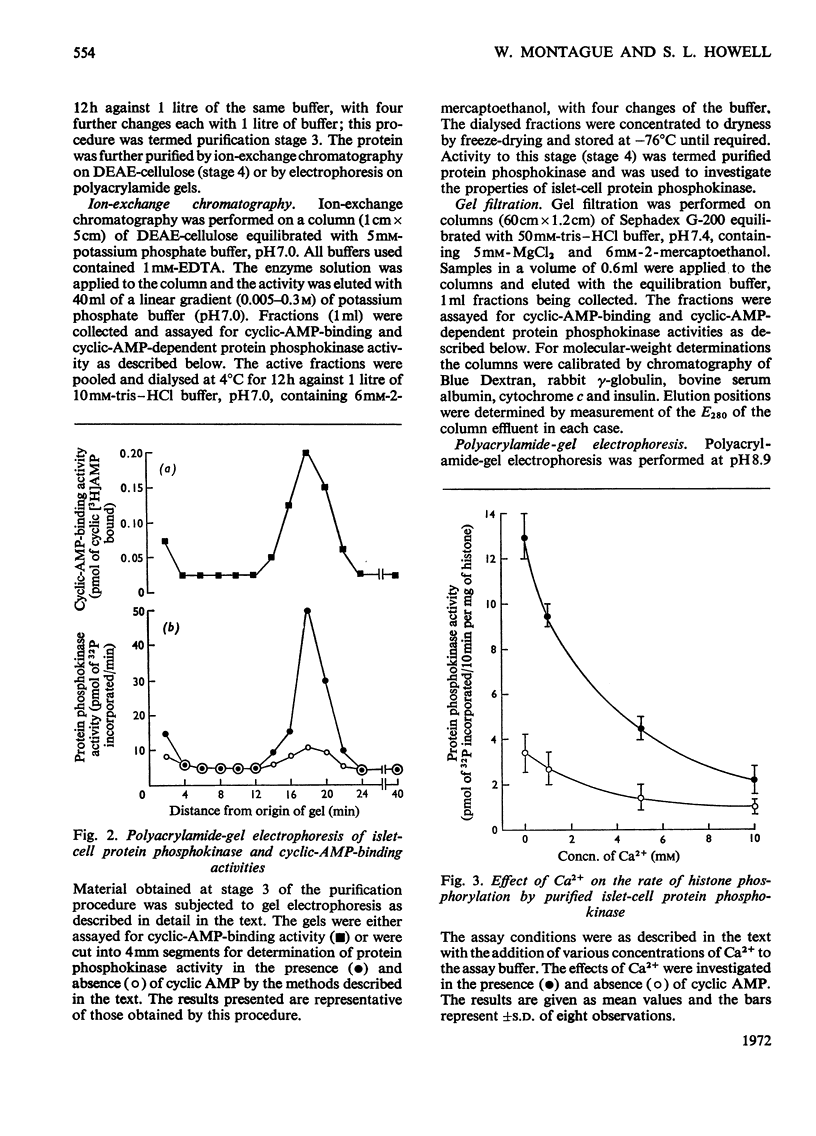
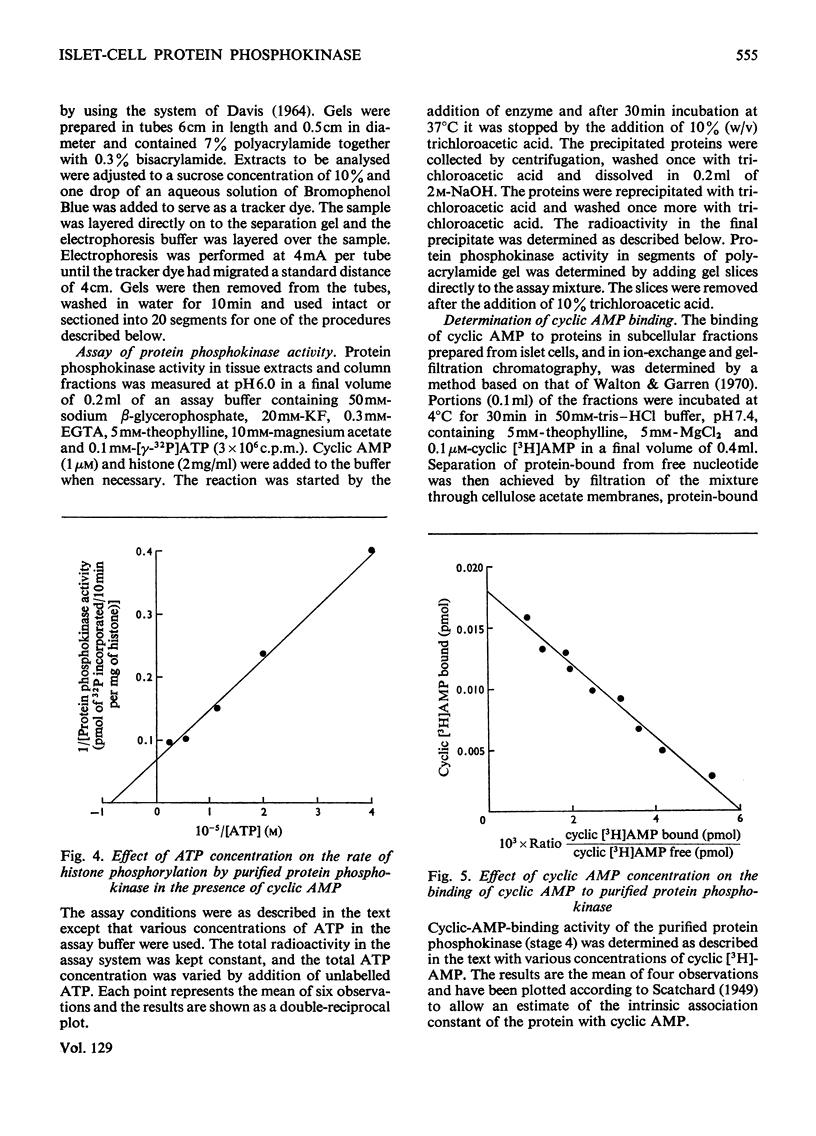
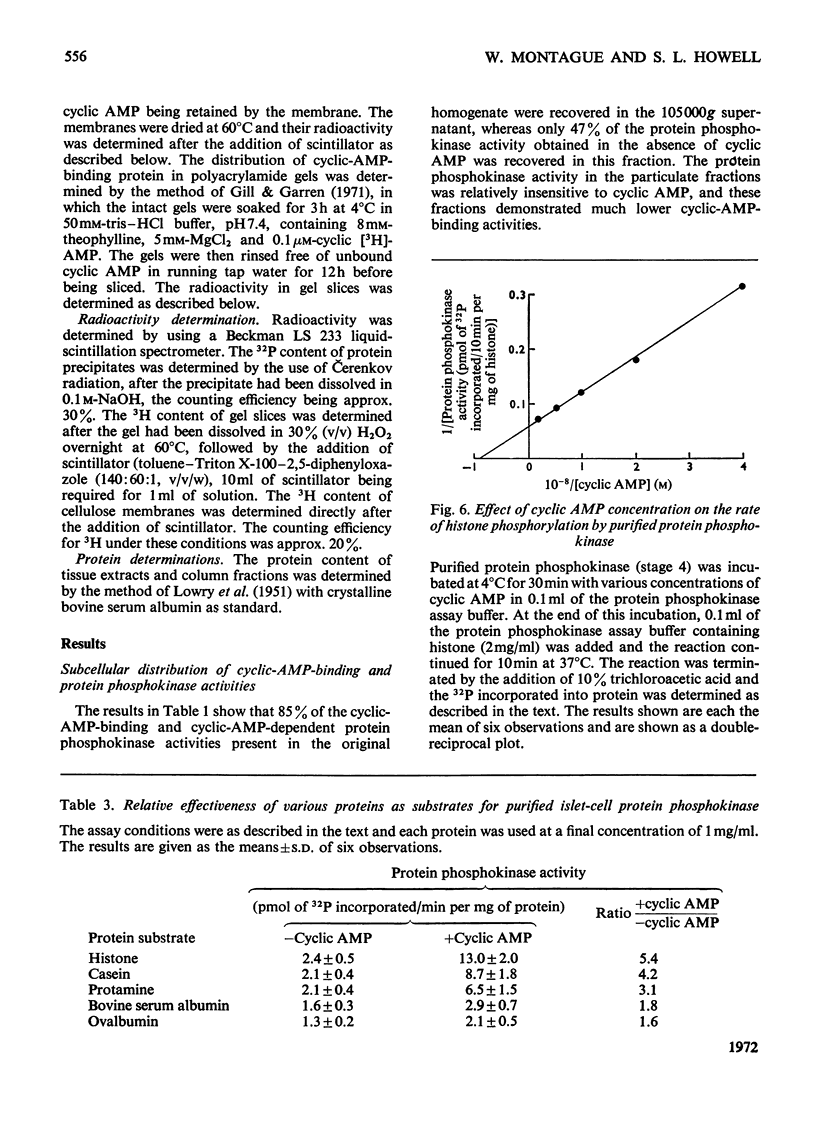
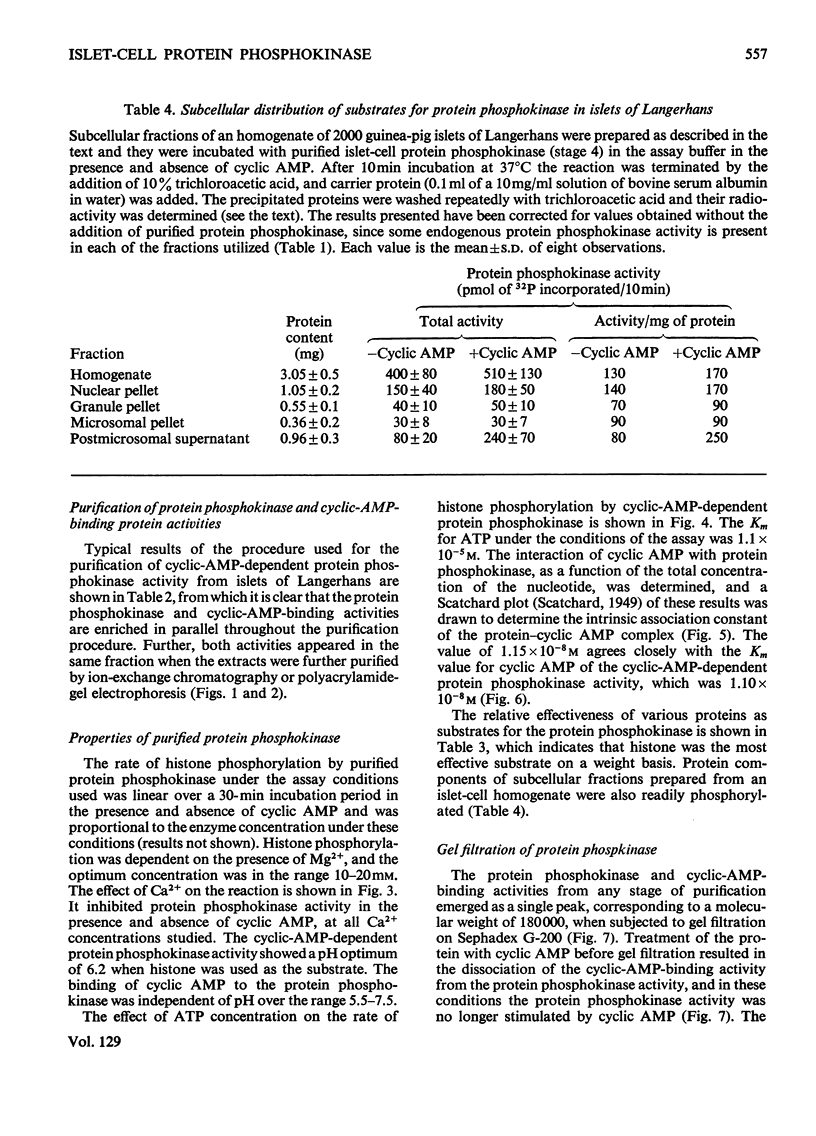
1. A protein was demonstrated in mammalian islets of Langerhans that after purification appeared as a single component possessing both cyclic-AMP (adenosine 3′:5′-cyclic monophosphate)-binding and cyclic-AMP-dependent protein phosphokinase activities. 2. The protein had an intrinsic association constant for cyclic AMP of 1.15×10−8m, which was similar to the Km for cyclic AMP (1.11×10−8m) of the protein phosphokinase activity. 3. Incubation of the protein in the presence of cyclic AMP resulted in its dissociation into cyclic-AMP-independent protein phosphokinase (catalytic) and cyclic-AMP-binding (receptor) subunits, which could be separated on Sephadex G-200. 4. The cyclic-AMP-dependent protein phosphokinase was capable of phosphorylating a variety of proteins, the most readily phosphorylated being histone, casein and protein components of sub-cellular fractions prepared from islets of Langerhans. 5. The cyclic-AMP-dependent phosphorylation of histone had a Km for ATP of 1.1×10−5m. 6. The endogenous protein phosphokinase activity in rat islets incubated with agents that are known to alter the intracellular concentration of cyclic AMP was investigated. Theophylline and 3-isobutyl-1-methylxanthine, agents that raise cyclic AMP concentrations in islets, increased the activity of the protein phosphokinase, whereas adrenaline, which lowers islet cyclic AMP concentrations, decreased its activity. 7. It is suggested that cyclic AMP may exert its effects on insulin release by increasing the activity of a protein phosphokinase and may thereby promote the phosphorylation and activity of a rate-determining component of the secretory mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher R. W., Ho R. J., Meng H. C., Sutherland E. W. Adenosine 3',5'-monophosphate in biological materials. II. The measurement of adenosine 3',5'-monophosphate in tissues and the role of the cyclic nucleotide in the lipolytic response of fat to epinephrine. J Biol Chem. 1965 Nov;240(11):4515–4523. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Erlichman J., Hirsch A. H., Rosen O. M. Interconversion of cyclic nucleotide-activated and cyclic nucleotide-independent forms of a protein kinase from beef heart. Proc Natl Acad Sci U S A. 1971 Apr;68(4):731–735. doi: 10.1073/pnas.68.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. A cyclic-3',5'-adenosine monophosphate dependent protein kinase from the adrenal cortex: comparison with a cyclic AMP binding protein. Biochem Biophys Res Commun. 1970 May 11;39(3):335–343. doi: 10.1016/0006-291x(70)90581-4. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. Role of the receptor in the mechanism of action of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):786–790. doi: 10.1073/pnas.68.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. B., Rasmussen H., DiBella F., Guthrow C. E., Jr Cyclic adenosine 3':5'-monophosphate-stimulated phosphorylation of isolated neurotubule subunits. Proc Natl Acad Sci U S A. 1970 Oct;67(2):652–659. doi: 10.1073/pnas.67.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Fink C. J., Lacy P. E. Isolation and properties of secretory granules from rat islets of Langerhans. I. Isolation of a secretory granule fraction. J Cell Biol. 1969 Apr;41(1):154–161. doi: 10.1083/jcb.41.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J. 1968 Jun;108(1):17–24. doi: 10.1042/bj1080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. 3. Purification and properties of adenosine 3',5'-monophosphate-dependent protein kinase from bovine brain. J Biol Chem. 1969 Dec 10;244(23):6395–6402. [PubMed] [Google Scholar]
- Montague W., Cook J. R. The role of adenosine 3':5'-cyclic monophosphate in the regulation of insulin release by isolated rat islets of Langerhans. Biochem J. 1971 Mar;122(1):115–120. doi: 10.1042/bj1220115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann E. M., Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Separation of regulatory and catalytic subunits of the cyclic 3',5'-adenosine monophosphate-dependent protein kinase(s) of rabbit skeletal muscle. Biochem Biophys Res Commun. 1971 Jan 22;42(2):187–194. doi: 10.1016/0006-291x(71)90086-6. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Walton G. M., Garren L. D. An assay for adenosine 3',5'-cyclic monophosphate based on the association of the nucleotide with a partially purified binding protein. Biochemistry. 1970 Oct 13;9(21):4223–4229. doi: 10.1021/bi00823a026. [DOI] [PubMed] [Google Scholar]


