Abstract
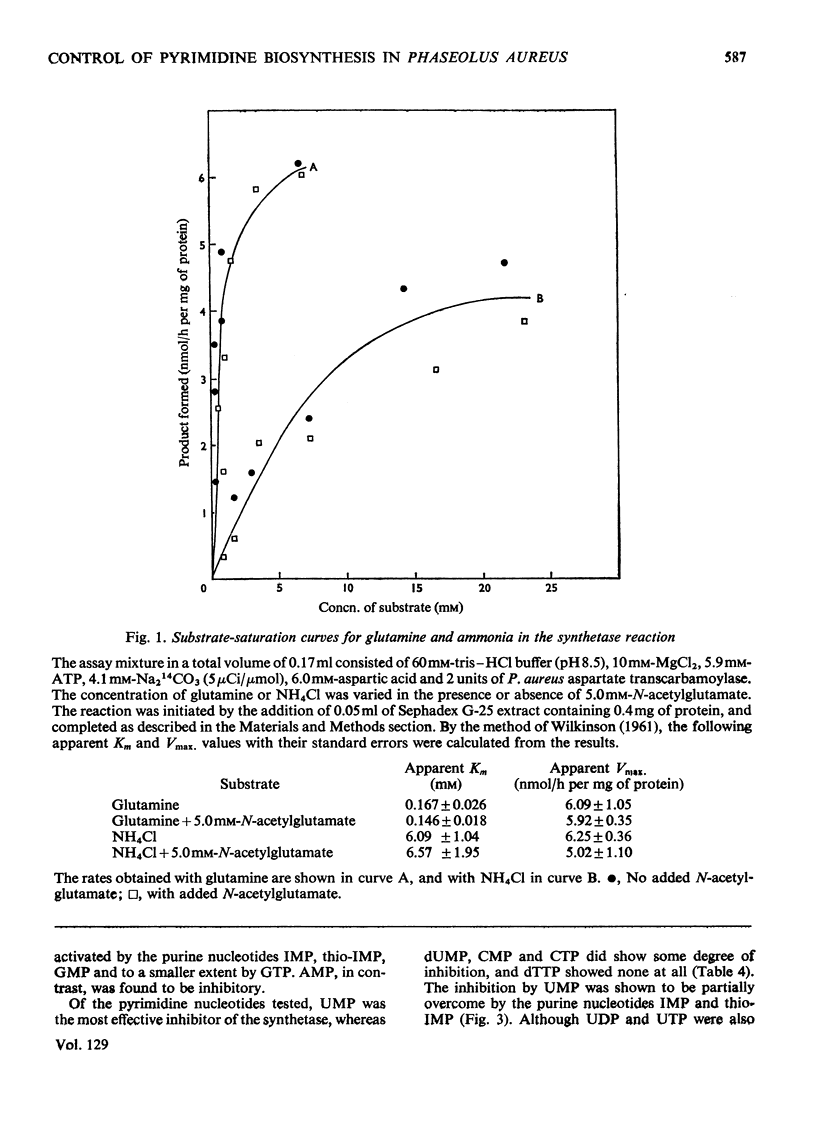
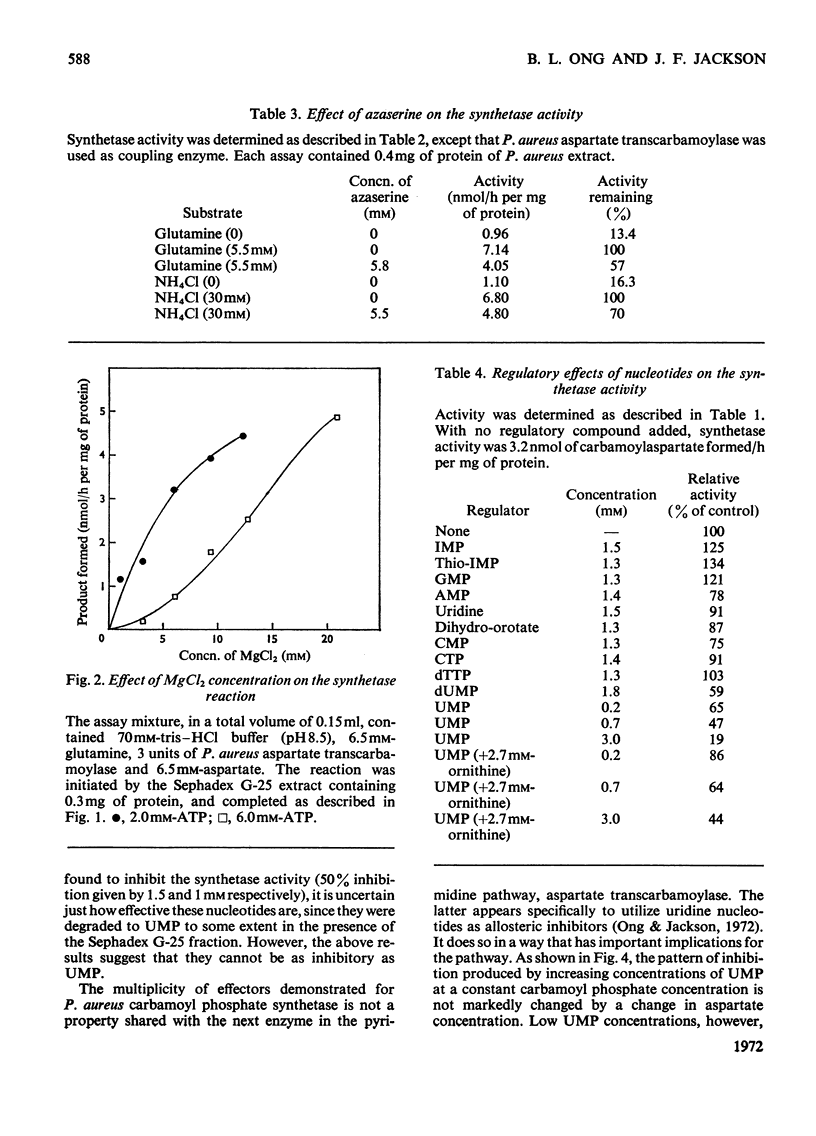
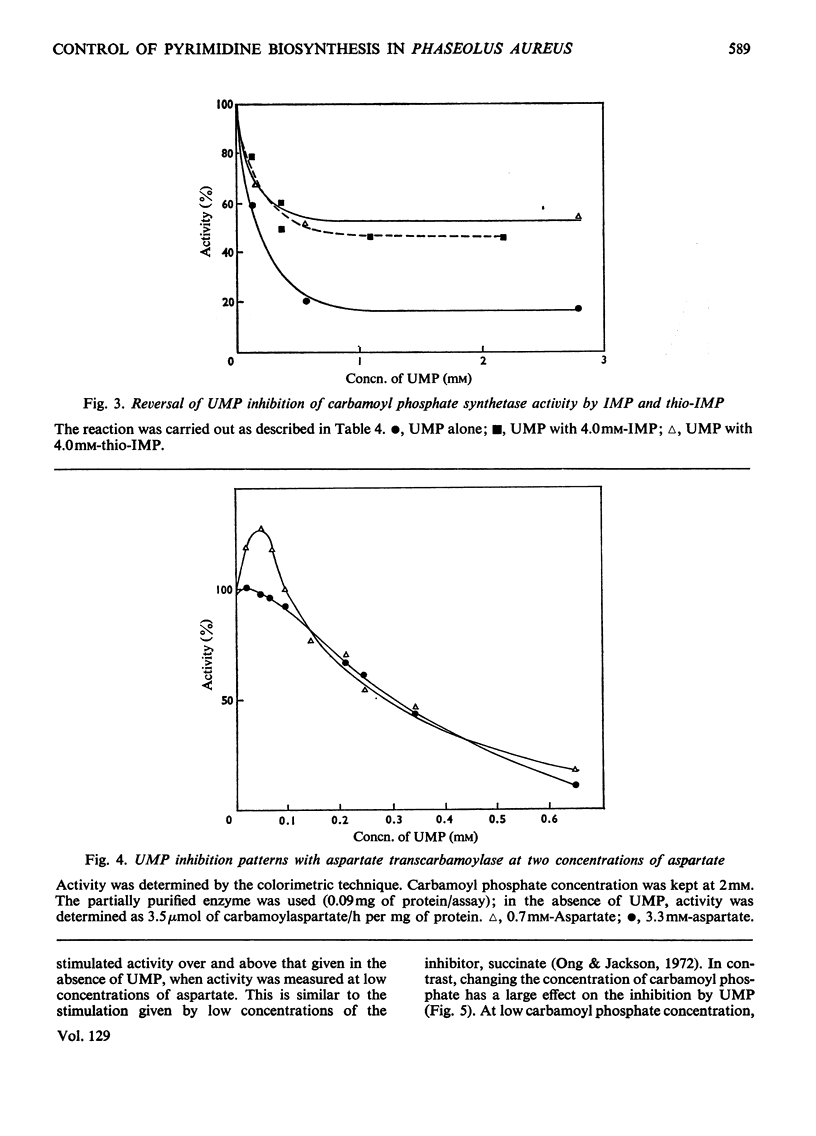
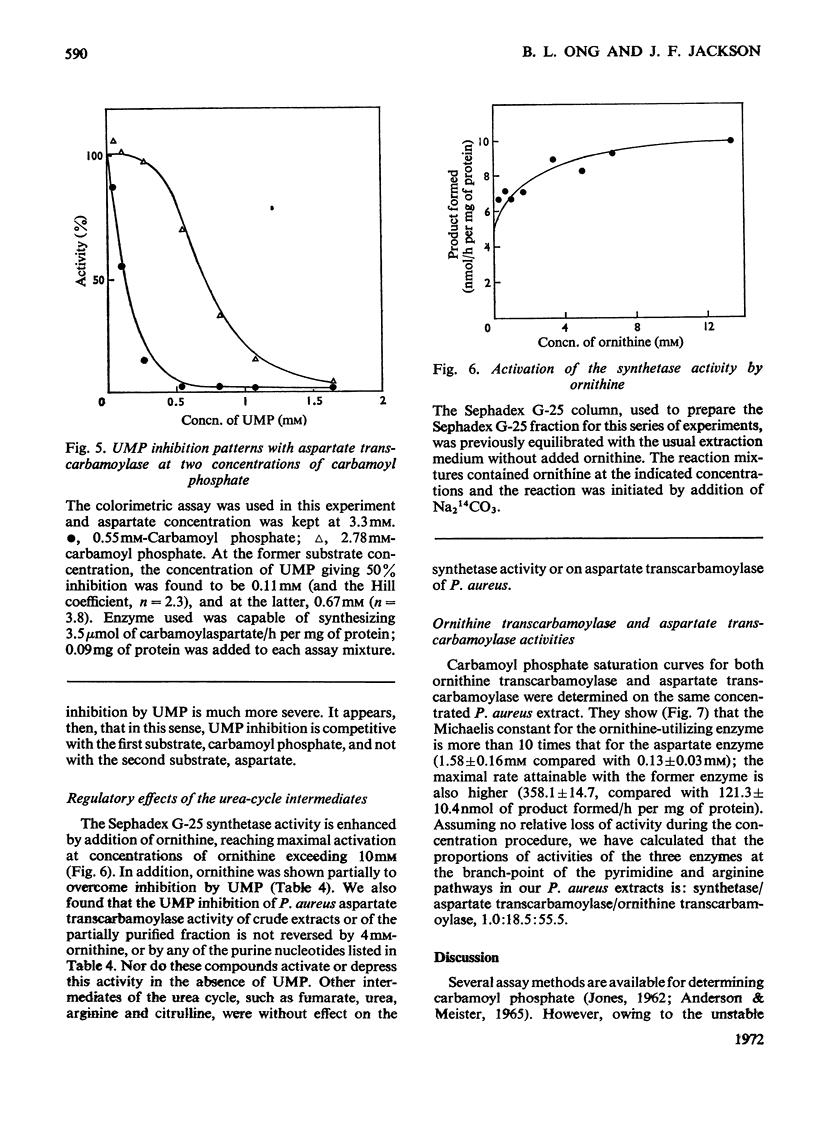
1. Carbamoyl phosphate synthetase activity of Phaseolus aureus extracts was assayed by coupling it to the catalytic subunit of Escherichia coli aspartate transcarbamoylase and determining the [14C]carbamoylaspartate so formed. The stability of the activity was improved by the addition of ornithine and dimethyl sulphoxide to the extraction medium. 2. The synthetase activity was found to utilize either glutamine or ammonia as amino donor, the Michaelis constants being 0.17±0.03mm and 6.1±1.0mm respectively. N-Acetylglutamate did not significantly alter the rate with either substrate, and azaserine inhibited the reaction with both amino donors to the same extent. 3. Ornithine was shown to stimulate the activity, and to counteract inhibition by UMP. The purine nucleotides IMP and GMP enhanced carbamoyl phosphate formation, whereas AMP had an inhibitory effect. 4. The Michaelis constant for carbamoyl phosphate was determined in concentrated extracts for both aspartate transcarbamoylase and ornithine transcarbamoylase activities, and was 0.13±0.03mm and 1.58±0.16mm respectively. The ratio of the activities of these two enzymes, determined at near-saturating substrate concentrations, was 1:3 (aspartate transcarbamoylase/ornithine transcarbamoylase). 5. It is concluded that in this plant tissue there is one enzyme, carbamoyl phosphate synthetase, supplying carbamoyl phosphate to both the pyrimidine and arginine pathways, that the pyrimidine pathway claims most of the available carbamoyl phosphate (depending on the concentration of the nucleotide effectors) when this intermediate is present at low concentrations; and that when the carbamoyl phosphate concentration is increased, possibly by ornithine stimulation, a larger proportion can be taken up by the arginine pathway.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN C. M., Jr, JONES M. E. DECOMPOSITION OF CARBAMYLPHOSPHATE IN AQUEOUS SOLUTIONS. Biochemistry. 1964 Sep;3:1238–1247. doi: 10.1021/bi00897a010. [DOI] [PubMed] [Google Scholar]
- ATKINSON M. R., JACKSON J. F., MORTON R. K. Nicotinamide mononucleotide adenylyltransferase of pig-liver nuclei. The effects of nicotinamide mononucleotide concentration and pH on dinucleotide synthesis. Biochem J. 1961 Aug;80:318–323. doi: 10.1042/bj0800318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. M., Marvin S. V. Effect of ornithine, IMP, and UMP on carbamyl phosphate synthetase from Escherichia coli. Biochem Biophys Res Commun. 1968 Sep 30;32(6):928–934. doi: 10.1016/0006-291x(68)90116-2. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Evidence for an activated form of carbon dioxide in the reaction catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1965 Dec;4(12):2803–2809. doi: 10.1021/bi00888a034. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Eagles J., Laird W. M., Matai S., Self R., Synge R. L. N-carbamoyl-2-(p-hydroxyphenyl)glycine from leaves of broad bean (Vicia faba L.). Biochem J. 1971 Feb;121(3):425–430. doi: 10.1042/bj1210425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Hager S. E., Jones M. E. Initial steps in pyrimidine synthesis in Ehrlich ascites carcinoma in vitro. II. The synthesis of carbamyl phosphate by a soluble, glutamine-dependent carbamyl phosphate synthetase. J Biol Chem. 1967 Dec 25;242(24):5667–5673. [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A. Enzymatic synthesis and breakdown of a pyrimidine, orotic acid. I. Dihydroortic acid, ureidosuccinic acid, and 5-carboxymethylhydantoin. J Biol Chem. 1954 Apr;207(2):911–924. [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Naylor A. W. Purine and pyrimidine nucleotide inhibition of carbamyl phosphate synthetase from pea seedlings. Biochem Biophys Res Commun. 1968 May 10;31(3):322–327. doi: 10.1016/0006-291x(68)90478-6. [DOI] [PubMed] [Google Scholar]
- O'Neal T. D., Naylor A. W. Partial purification and properties of carbamoyl phosphate synthetase of Alaska pea (Pisum sativum L. cultivar Alaska). Biochem J. 1969 Jun;113(2):271–279. doi: 10.1042/bj1130271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Aspartate transcarbamoylase from Phaseolus aureus. Partial purification and properties. Biochem J. 1972 Sep;129(3):571–581. doi: 10.1042/bj1290571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Determination of aspartate transcarbamylase by the radioassay of carbamyl 14C-aspartate separated by high-voltage paper electrophoresis. Anal Biochem. 1971 Jul;42(1):289–293. doi: 10.1016/0003-2697(71)90039-x. [DOI] [PubMed] [Google Scholar]
- Piérard A. Control of the activity of Escherichia coli carbamoyl phosphate synthetase by antagonistic allosteric effectors. Science. 1966 Dec 23;154(3756):1572–1573. doi: 10.1126/science.154.3756.1572. [DOI] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- RAVEL J. M., GRONA M. L., HUMPHREYS J. S., SHIVE W. Properties and biotin content of purified preparations of the ornithinecitrulline enzyme of Streptococcus lactis. J Biol Chem. 1959 Jun;234(6):1452–1455. [PubMed] [Google Scholar]
- ROGERS P., NOVELLI G. D. Purification of ornithine transcarbamylase from derepressed cells of Escherichia coli W. Arch Biochem Biophys. 1962 Feb;96:398–407. doi: 10.1016/0003-9861(62)90426-5. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Ito K. Control of pyrimidine biosynthesis in mammalian tissues. I. Partial purification and characterization of glutamine-utilizing carbamyl phosphate synthetase of mouse spleen and its tissue distribution. J Biol Chem. 1969 Oct 10;244(19):5403–5413. [PubMed] [Google Scholar]
- Tramell P. R., Campbell J. W. Carbamyl phosphate synthesis in a land snail, Strophocheilus oblongus. J Biol Chem. 1970 Dec 25;245(24):6634–6641. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharia O., Soru E. Staphylococcal ornithine carbamoyltransferase. Purification and some properties. Eur J Biochem. 1971 Jan 1;18(1):28–34. doi: 10.1111/j.1432-1033.1971.tb01210.x. [DOI] [PubMed] [Google Scholar]


