Abstract
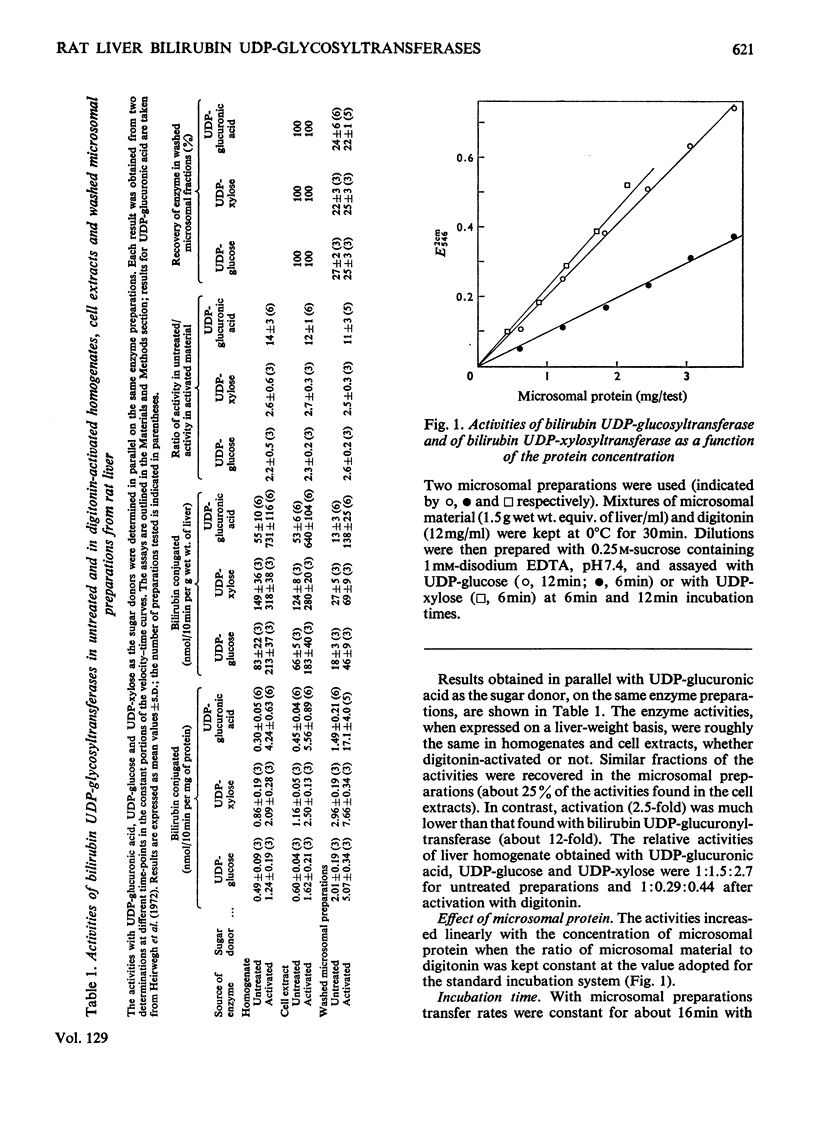
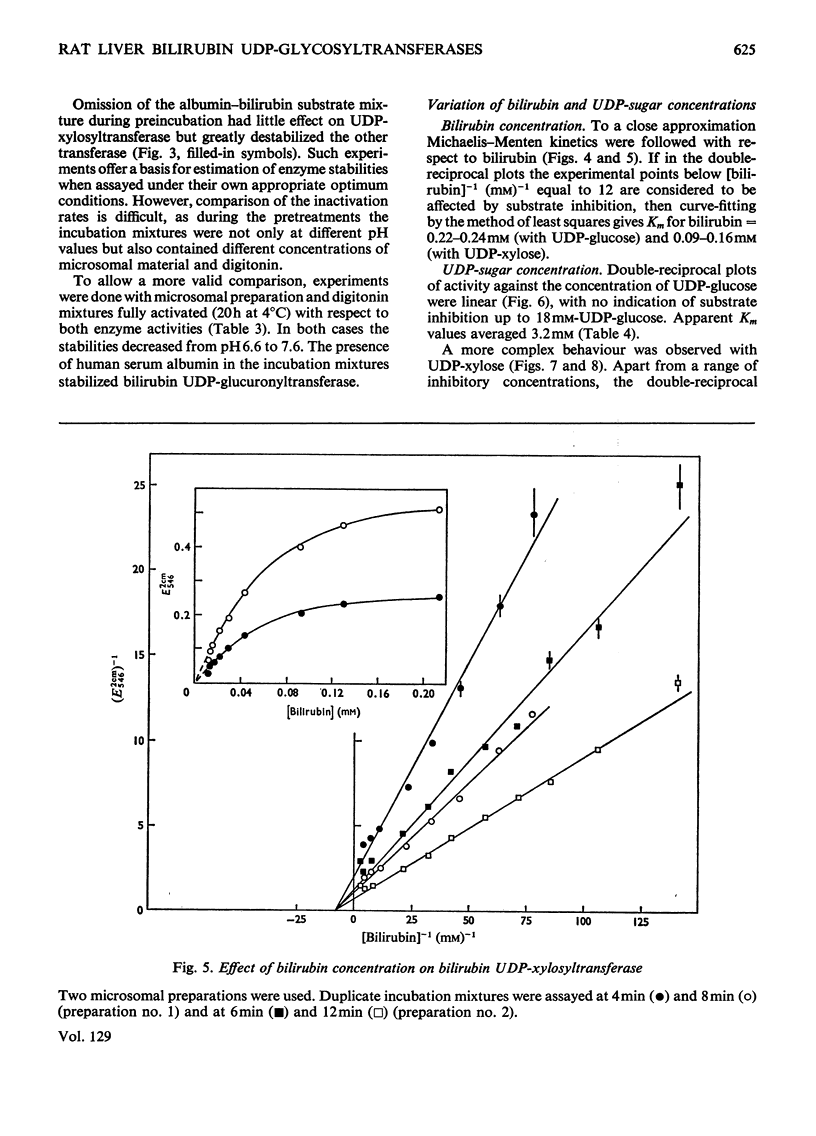
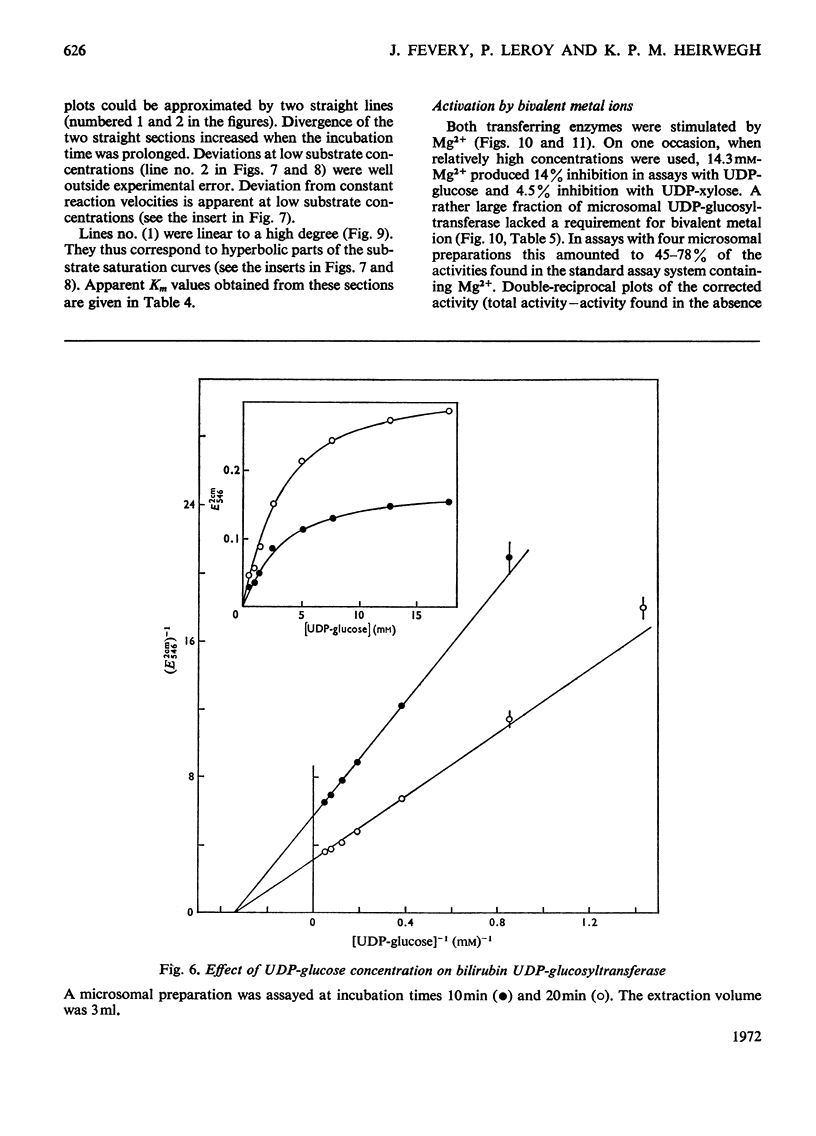
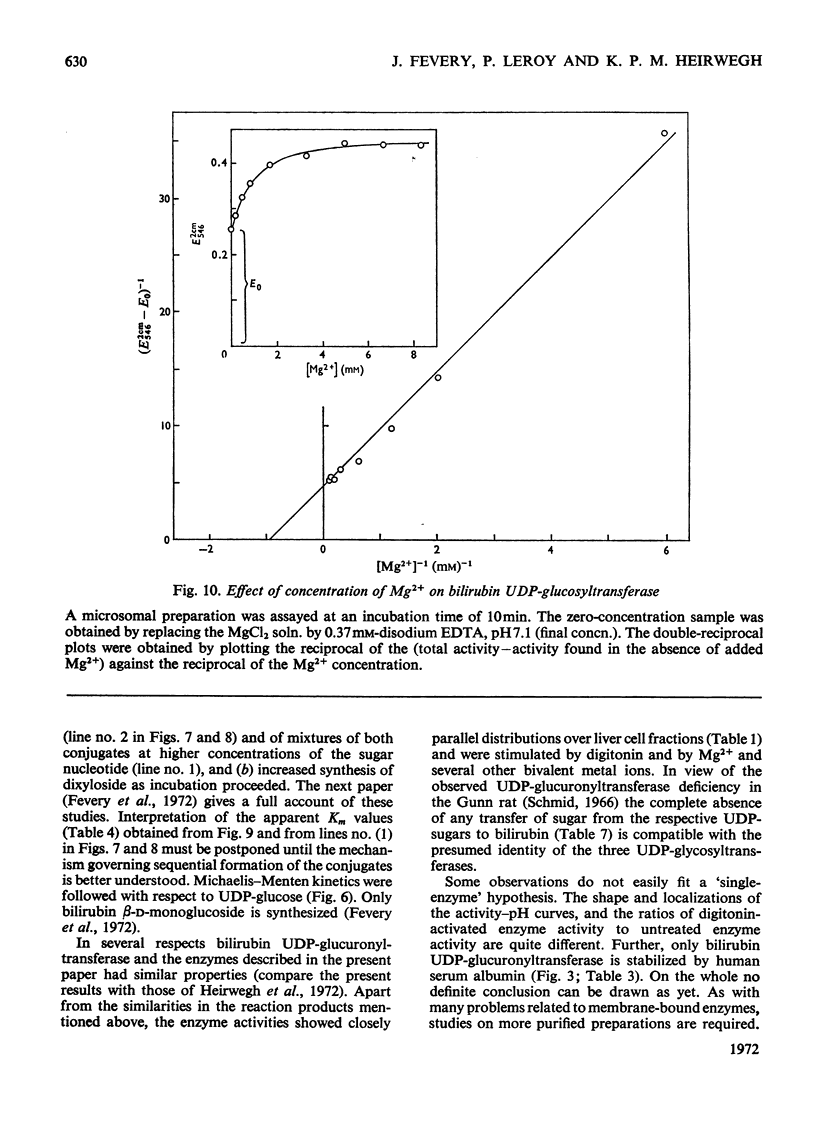
1. Digitonin-treated and untreated homogenates, cell extracts and washed microsomal preparations from liver of Wistar R rats are capable of transferring sugar from UDP-glucose or UDP-xylose to bilirubin. No formation of bilirubin glycosides occurred with UDP-galactose or d-glucose, d-xylose or d-glucuronic acid as the sources of sugar. 2. Procedures to assay digitonin-activated and unactivated bilirubin UDP-glucosyltransferase and bilirubin UDP-xylosyltransferase were developed. 3. In digitonin-activated microsomal preparations the transferring enzymes had the following properties. Both enzyme activities were increased 2.5-fold by pretreatment with digitonin. They were optimum at pH6.6–7.2. Michaelis–Menten kinetics were followed with respect to UDP-glucose. In contrast, double-reciprocal plots of enzyme activity against the concentration of UDP-xylose showed two intersecting straight-line sections corresponding to concentration ranges where either bilirubin monoxyloside was formed (at low UDP-xylose concentrations) or where mixtures of both the mono- and di-xyloside were synthesized (at high UDP-xylose concentrations). Both enzyme activities were stimulated by Mg2+; Ca2+ was slightly less, and Mn2+ slightly more, stimulatory than Mg2+. Of the activities found in standard assay systems containing Mg2+, 58–78% (substrate UDP-glucose) and 0–38% (substrate UDP-xylose) were independent of added bivalent metal ion. Double-reciprocal plots of the Mg2+-dependent activities against the concentration of added Mg2+ were linear. 4. In comparative experiments the relative activities of liver homogenates obtained with UDP-glucuronic acid, UDP-glucose and UDP-xylose were 1:1.5:2.7 for untreated preparations and 1:0.29:0.44 after activation with digitonin. 5. Bilirubin UDP-glucuronyltransferase was protected against denaturation by human serum albumin, whereas bilirubin UDP-xylosyltransferase was not. 6. Digitonin-treated and untreated liver homogenates from Gunn rats were inactive in transferring sugar to bilirubin from UDP-glucuronic acid (in agreement with the work of others), UDP-glucose or UDP-xylose.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwood D., Graham A. B., Wood G. C. The phospholipid-dependence of uridine diphosphate glucuronyltransferase. Reactivation of phospholipase A-inactivated enzyme by phospholipids and detergents. Biochem J. 1971 Aug;123(5):875–882. doi: 10.1042/bj1230875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- Bdolah A., Feingold D. S. Decarboxylation of uridine diphosphate-D-glucuronic acid by an enzyme preparation from hen oviduct. Biochem Biophys Res Commun. 1965 Dec 21;21(6):543–546. doi: 10.1016/0006-291x(65)90519-x. [DOI] [PubMed] [Google Scholar]
- Collins D. C., Jirku H., Layne D. S. Steroid N-acetylglucosaminyl transferase. Localization and some properties of the enzyme in rabbit tissues. J Biol Chem. 1968 Jun 10;243(11):2928–2933. [PubMed] [Google Scholar]
- Collins D. C., Williamson D. G., Layne D. S. Steroid glucosides. Enzymatic synthesis by a partially purified transferase from rabbit liver microsomes. J Biol Chem. 1970 Feb 25;245(4):873–876. [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Fevery J., Heirwegh K. P. Mass-spectrometric structure elucidation of dog bile azopigments as the acyl glycosides of glucopyranose and xylopyranose. Biochem J. 1971 Dec;125(3):811–819. doi: 10.1042/bj1250811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAUGHADAY W. H., LOWRY O. H., ROSEBROUGH N. J., FIELDS W. S. Determination of cerebrospinal fluid protein with the Folin phenol reagent. J Lab Clin Med. 1952 Apr;39(4):663–665. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton G. J. Uridine diphosphate glucose and the synthesis of phenolic glucosides by mollusks. Arch Biochem Biophys. 1966 Sep 26;116(1):399–405. doi: 10.1016/0003-9861(66)90046-4. [DOI] [PubMed] [Google Scholar]
- Fevery J., Leroy P., Van de Vijver M., Heirwegh K. P. Structures of bilirubin conjugates synthesized in vitro from bilirubin and uridine diphosphate glucuronic acid, uridine diphosphate glucose or uridine diphosphate xylose by preparations from rat liver. Biochem J. 1972 Sep;129(3):635–644. doi: 10.1042/bj1290635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevery J., Van Hees G. P., Leroy P., Compernolle F., Heirwegh K. P. Excretion in dog bile of glucose and xylose conjugates of bilirubin. Biochem J. 1971 Dec;125(3):803–810. doi: 10.1042/bj1250803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebner E. E., Hall C. W., Neufeld E. F. Glycosylation of serine residues by a uridine diphosphate-xylose: protein xylosyltransferase from mouse mastocytoma. Arch Biochem Biophys. 1966 Sep 26;116(1):391–398. doi: 10.1016/0003-9861(66)90045-2. [DOI] [PubMed] [Google Scholar]
- Grebner E. E., Hall C. W., Neufeld E. F. Incorporation of D-xylose-C14 into glycoprotein by particles from hen oviduct. Biochem Biophys Res Commun. 1966 Mar 22;22(6):672–677. doi: 10.1016/0006-291x(66)90199-9. [DOI] [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Leroy P., Van Roy F. P., Jansen F. H. Heterogeneity of bile pigment conjugates as revealed by chromatography of their ethyl anthranilate azopigments. Biochem J. 1970 Dec;120(4):877–890. doi: 10.1042/bj1200877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirwegh K. P., Van de Vijver M., Fevery J. Assay and properties of dititonin-activated bilirubin uridine diphosphate glucuronyltransferase from rat liver. Biochem J. 1972 Sep;129(3):605–618. doi: 10.1042/bj1290605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman B., Basu S., Roseman S. Enzymatic synthesis of disialogangliosides from monosialogangliosides by sialyltransferases from embryonic chicken brain. J Biol Chem. 1968 Nov 10;243(21):5804–5807. [PubMed] [Google Scholar]
- Keppler D., Rudigier J., Decker K. Enzymic determination of uracil nucleotides in tissues. Anal Biochem. 1970 Nov;38(1):105–114. doi: 10.1016/0003-2697(70)90160-0. [DOI] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert J. E., DeLuca S. The synthesis of uridine diphosphate xylose by particulate preparations from mouse mast-cell tumors. Biochim Biophys Acta. 1967 Jun 13;141(1):193–196. doi: 10.1016/0304-4165(67)90263-2. [DOI] [PubMed] [Google Scholar]
- Van Damme B., Fevery J., Heirwegh K. P. Altered composition of bilirubin conjugates in rat bile after obstruction of the common bile duct. Experientia. 1971 Jan 15;27(1):27–28. doi: 10.1007/BF02137721. [DOI] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F. P., Meuwissen J. A., De Meuter F., Heirwegh K. P. Determination of bilirubin in liver homogenates and serum with diazotized p-iodoaniline. Clin Chim Acta. 1971 Jan;31(1):109–118. doi: 10.1016/0009-8981(71)90367-6. [DOI] [PubMed] [Google Scholar]
- Williamson D. G., Polakova A., Layne D. S. Estrogen glucosides and galactosides: formation by rabbit liver microsomes in vitro. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1057–1062. doi: 10.1016/0006-291x(71)90011-8. [DOI] [PubMed] [Google Scholar]
- Wong K. P. Formation of bilirubin glucoside. Biochem J. 1971 Dec;125(4):929–934. doi: 10.1042/bj1250929a. [DOI] [PMC free article] [PubMed] [Google Scholar]


