Abstract
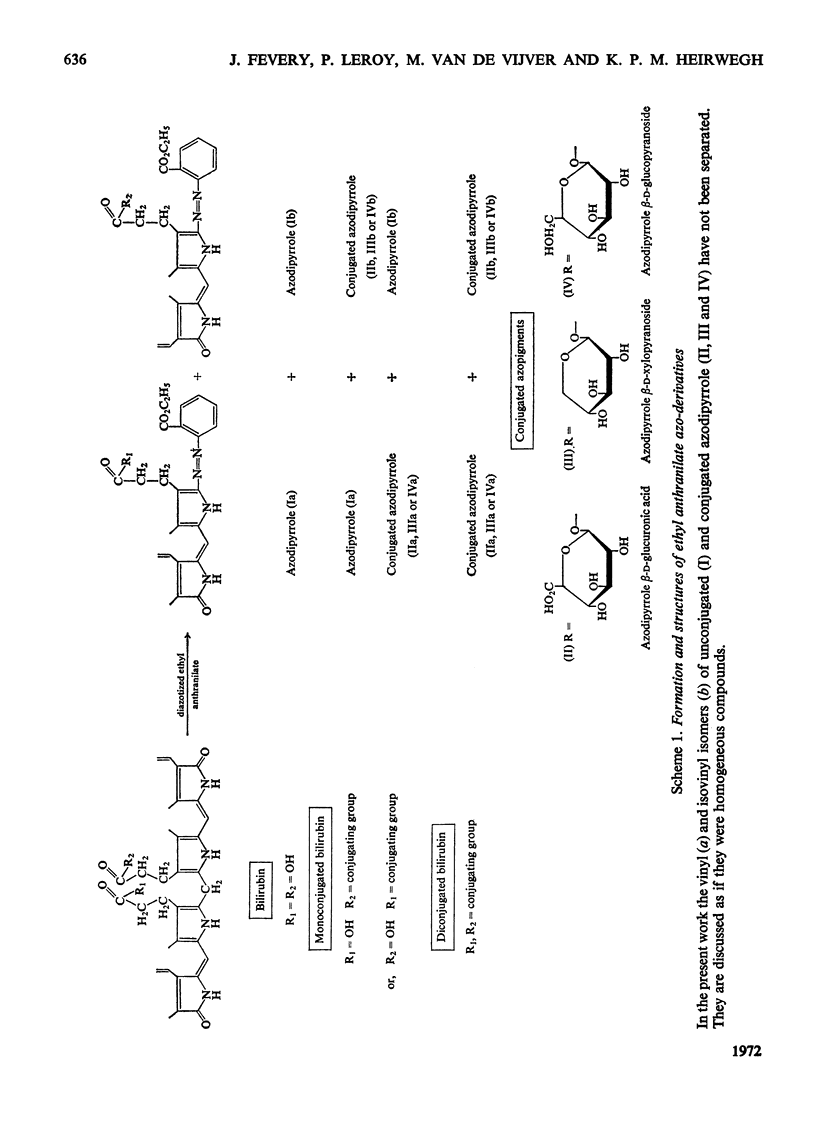
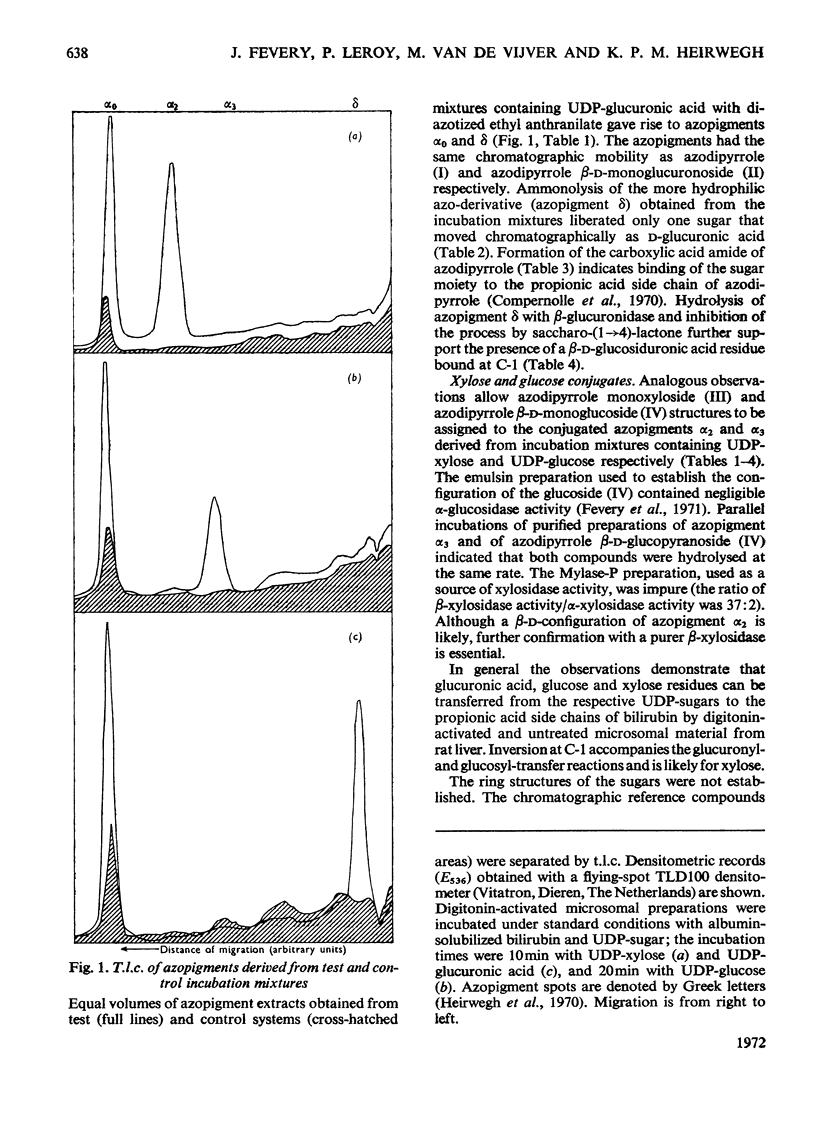
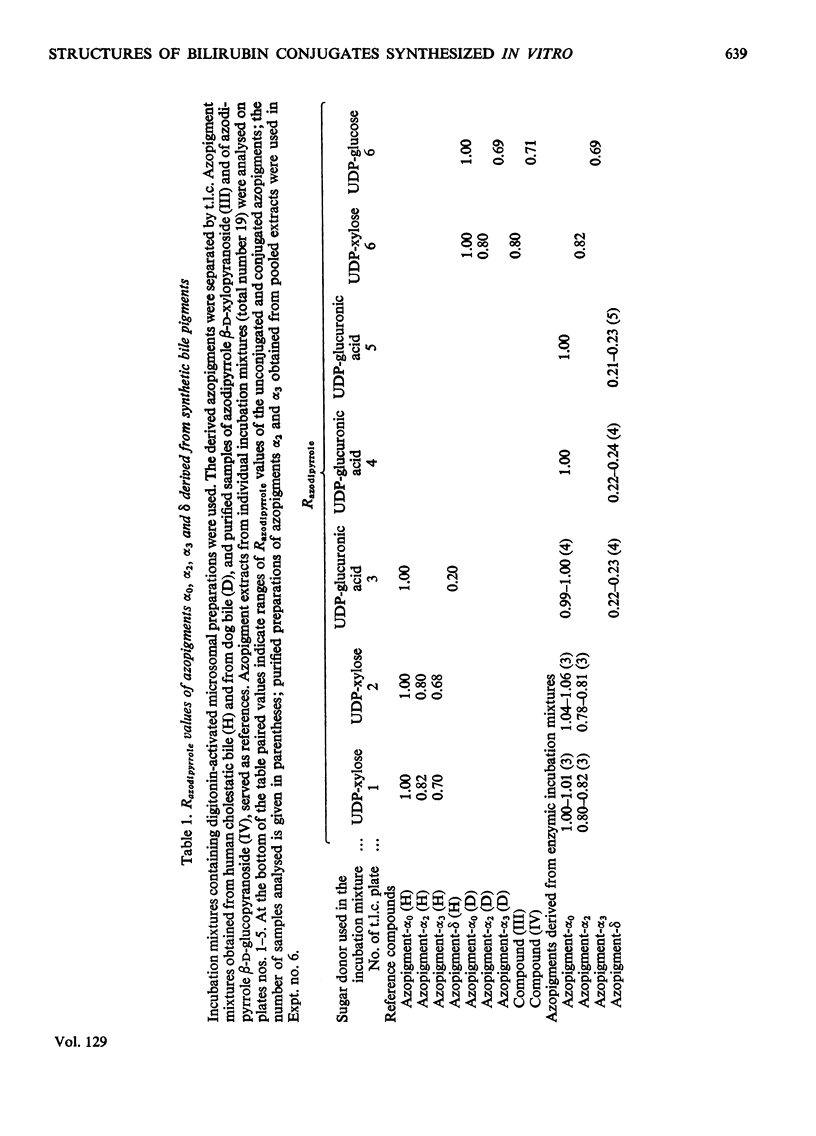
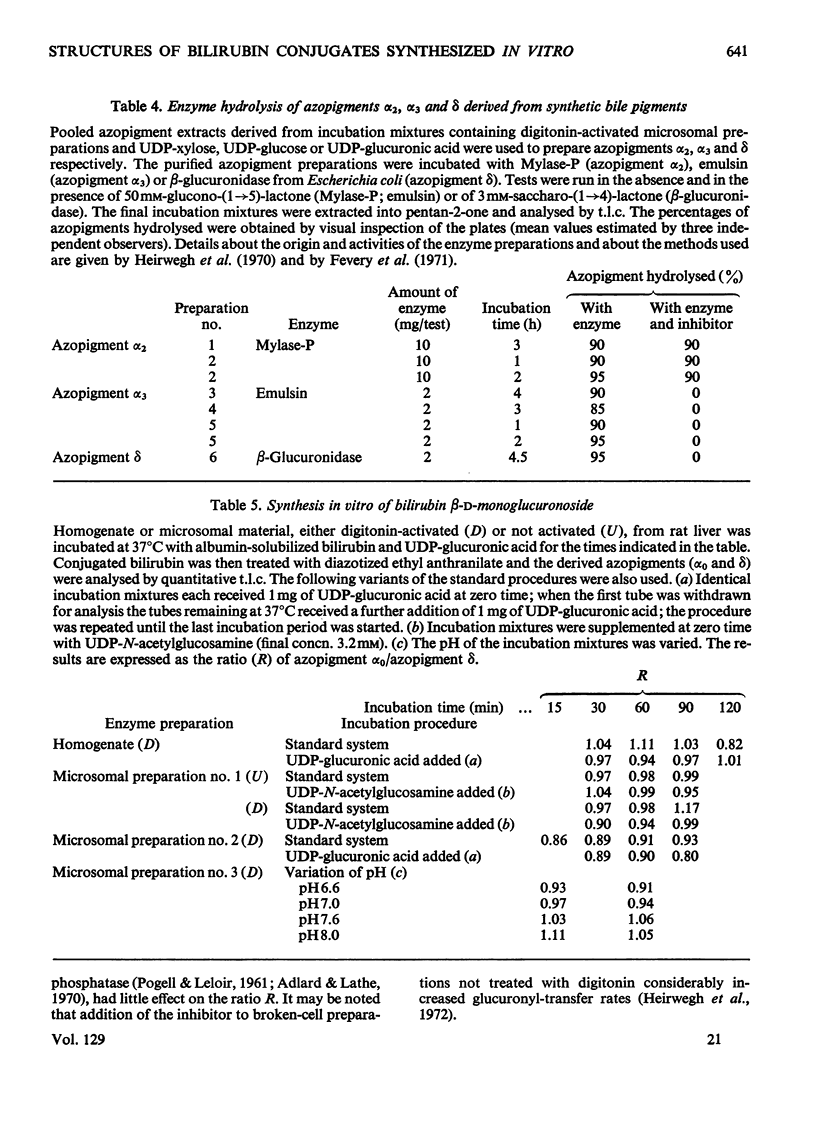
1. In incubation mixtures containing digitonin-activated or untreated preparations from rat liver, albumin-solubilized bilirubin as the acceptor substrate and (a) UDP-glucuronic acid, (b) UDP-glucose or (c) UDP-xylose as the sugar donor, formation of the following ester glycosides was demonstrated: with (a), bilirubin β-d-monoglucuronoside, with (b), bilirubin β-d-monoglucoside and with (c), bilirubin monoxyloside or mixtures of the mono-and di-xyloside. 2. With UDP-glucuronic acid prolonged incubation and variation of the composition of the incubation mixtures yielded equimolar amounts of azodipyrrole (I) and azodipyrrole β-d-monoglucuronoside (II) after treatment of the incubation mixtures with the diazonium salt of ethyl anthranilate. The azo-derivatives were identified by t.l.c. by reference to known compounds and by the following chemical tests. After ammonolysis the conjugated azo-derivative (II) yielded d-glucuronic acid and the carboxylic acid amide of azodipyrrole, indicating transfer of a glucuronic acid residue to the carboxylic acid groups of bilirubin. The β-d-configuration of the sugar moiety and binding at C-1 were demonstrated by enzymic hydrolysis tests. 3. Analogous evidence established the structure of the reaction product obtained with UDP-glucose as the sugar donor, as bilirubin β-d-monoglucoside. 4. With UDP-xylose as the sugar donor xylosyl transfer to the carboxylic acid groups of bilirubin with attachment at C-1 was demonstrated in an analogous way. A β-d-configuration is considered very likely, but requires confirmation. 5. Monoxyloside formation was predominant at pH7.4, whereas at decreasing pH values increasing fractions of the substrate were converted into the dixyloside. Prolonged incubation, low concentrations of bilirubin and high concentrations of UDP-xylose favoured diconjugate formation. The available evidence supports the synthesis sequence: bilirubin → bilirubin monoxyloside → bilirubin dixyloside.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlard B. P., Lathe G. H. The effect of steroids and nucleotidesoon solubilized bilirubin uridine diphosphate-glucuronyltransferase. Biochem J. 1970 Sep;119(3):437–445. doi: 10.1042/bj1190437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN A. K., ZUELZER W. W. Studies on the neonatal development of the glucuronide conjugating system. J Clin Invest. 1958 Mar;37(3):332–340. doi: 10.1172/JCI103613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M., Billing B. H., Heirwegh K. P. Determination of bilirubin UDP-glucuronyl transferase activity in needle-biopsy specimens of human liver. Clin Chim Acta. 1970 Jul;29(1):27–35. doi: 10.1016/0009-8981(70)90216-0. [DOI] [PubMed] [Google Scholar]
- Compernolle F., Jansen F. H., Heirwegh K. P. Mass-spectrometric study of the azopigments obtained from bile pigments with diazotized ethyl anthranilate. Biochem J. 1970 Dec;120(4):891–894. doi: 10.1042/bj1200891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Fevery J., Heirwegh K. P. Mass-spectrometric structure elucidation of dog bile azopigments as the acyl glycosides of glucopyranose and xylopyranose. Biochem J. 1971 Dec;125(3):811–819. doi: 10.1042/bj1250811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevery J., Leroy P., Heirwegh K. P. Enzymic transfer of glucose and xylose from uridine diphosphate glucose and uridine diphosphate xylose to bilirubin by untreated and digitonin-activated preparations from rat liver. Biochem J. 1972 Sep;129(3):619–633. doi: 10.1042/bj1290619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevery J., Van Hees G. P., Leroy P., Compernolle F., Heirwegh K. P. Excretion in dog bile of glucose and xylose conjugates of bilirubin. Biochem J. 1971 Dec;125(3):803–810. doi: 10.1042/bj1250803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., CARBONE J. V. The synthesis of bilirubin glucuronide by tissue homogenates. J Biol Chem. 1957 May;226(1):449–458. [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Leroy P., Van Roy F. P., Jansen F. H. Heterogeneity of bile pigment conjugates as revealed by chromatography of their ethyl anthranilate azopigments. Biochem J. 1970 Dec;120(4):877–890. doi: 10.1042/bj1200877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirwegh K. P., Van de Vijver M., Fevery J. Assay and properties of dititonin-activated bilirubin uridine diphosphate glucuronyltransferase from rat liver. Biochem J. 1972 Sep;129(3):605–618. doi: 10.1042/bj1290605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen F. H., Stoll M. S. Separation and structural analysis of vinyl- and isovinyl-azobilirubin derivatives. Biochem J. 1971 Nov;125(2):585–597. doi: 10.1042/bj1250585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATHE G. H., WALKER M. The synthesis of bilirubin glucuronide in animal and human liver. Biochem J. 1958 Dec;70(4):705–712. doi: 10.1042/bj0700705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow J. D., Murphy N. H. Isolation and properties of conjugated bilirubin from bile. Biochem J. 1970 Nov;120(2):311–327. doi: 10.1042/bj1200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGELL B. M., LELOIR L. F. Nucleotide activation of liver microsomal glucuronidation. J Biol Chem. 1961 Feb;236:293–298. [PubMed] [Google Scholar]
- Rugstad H. E., Robinson S. H., Yannoni C., Tashjian A. H., Jr Metabolism of bilirubin by a clonal strain of rat hepatoma cells. J Cell Biol. 1970 Dec;47(3):703–710. doi: 10.1083/jcb.47.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID R., HAMMAKER L., AXELROD J. The enzymatic formation of bilirubin glucuronide. Arch Biochem Biophys. 1957 Jul;70(1):285–288. doi: 10.1016/0003-9861(57)90103-0. [DOI] [PubMed] [Google Scholar]
- SCHOENFIELD L. J., BOLLMAN J. L. Further studies on the nature and source of the conjugated bile pigments. Proc Soc Exp Biol Med. 1963 Apr;112:929–932. doi: 10.3181/00379727-112-28213. [DOI] [PubMed] [Google Scholar]
- Van Damme B., Fevery J., Heirwegh K. P. Altered composition of bilirubin conjugates in rat bile after obstruction of the common bile duct. Experientia. 1971 Jan 15;27(1):27–28. doi: 10.1007/BF02137721. [DOI] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. P. Bilirubin glucuronyltransferase. Specific assay and kinetic studies. Biochem J. 1971 Nov;125(1):27–35. doi: 10.1042/bj1250027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. P. Formation of bilirubin glucoside. Biochem J. 1971 Dec;125(4):929–934. doi: 10.1042/bj1250929a. [DOI] [PMC free article] [PubMed] [Google Scholar]


