Abstract
1. Collagen was extracted from chick skin with dilute acetic acid followed by dilute acetic acid containing pepsin. 2. The solubilized collagens were purified and portions subjected to further digestion by pepsin. 3. This treatment decreased the aldehyde content but contamination by hexosamine was not diminished. 4. Pepsin treatment converted practically all the acid-soluble collagen into monomeric subunits (α-chains), but the pepsinsolubilized material retained a significant amount of higher subunits (β- and γ-chains). 5. Treatment lowered the rate of fibrillogenesis by acid-soluble collagen, but was without effect on pepsin-solubilized collagen.
Full text
PDF

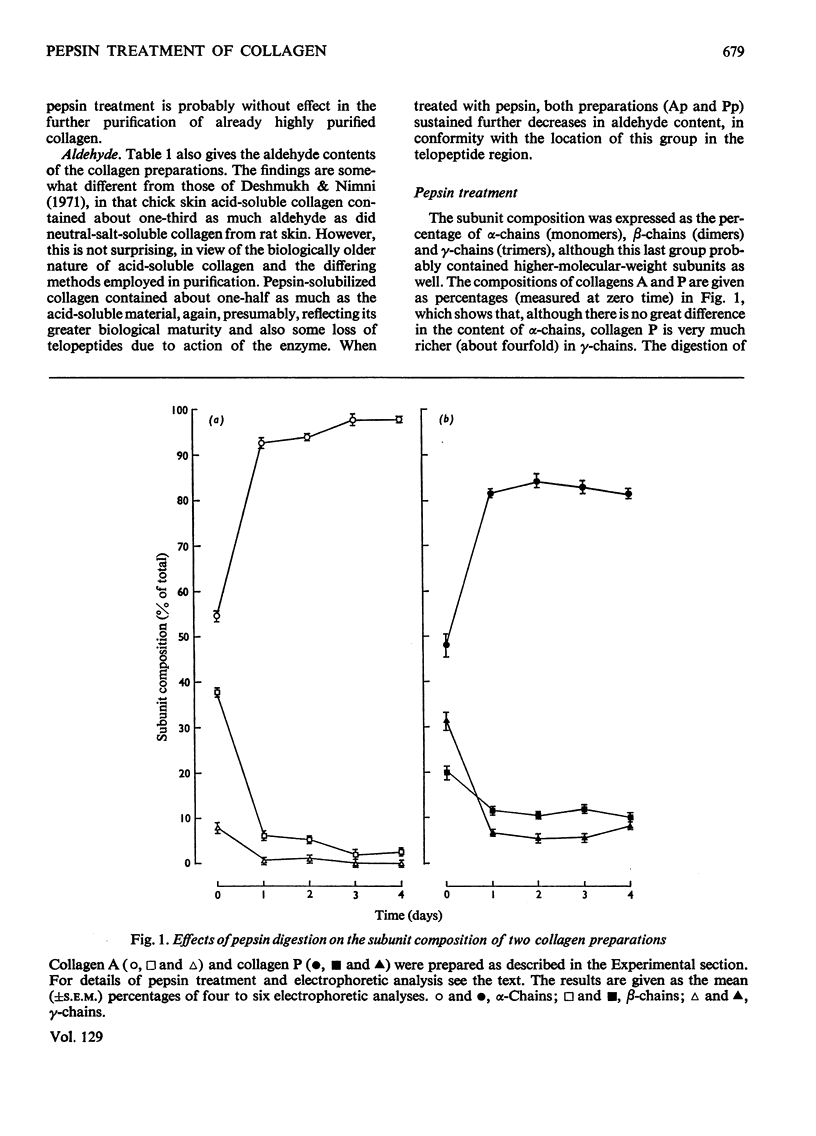

Selected References
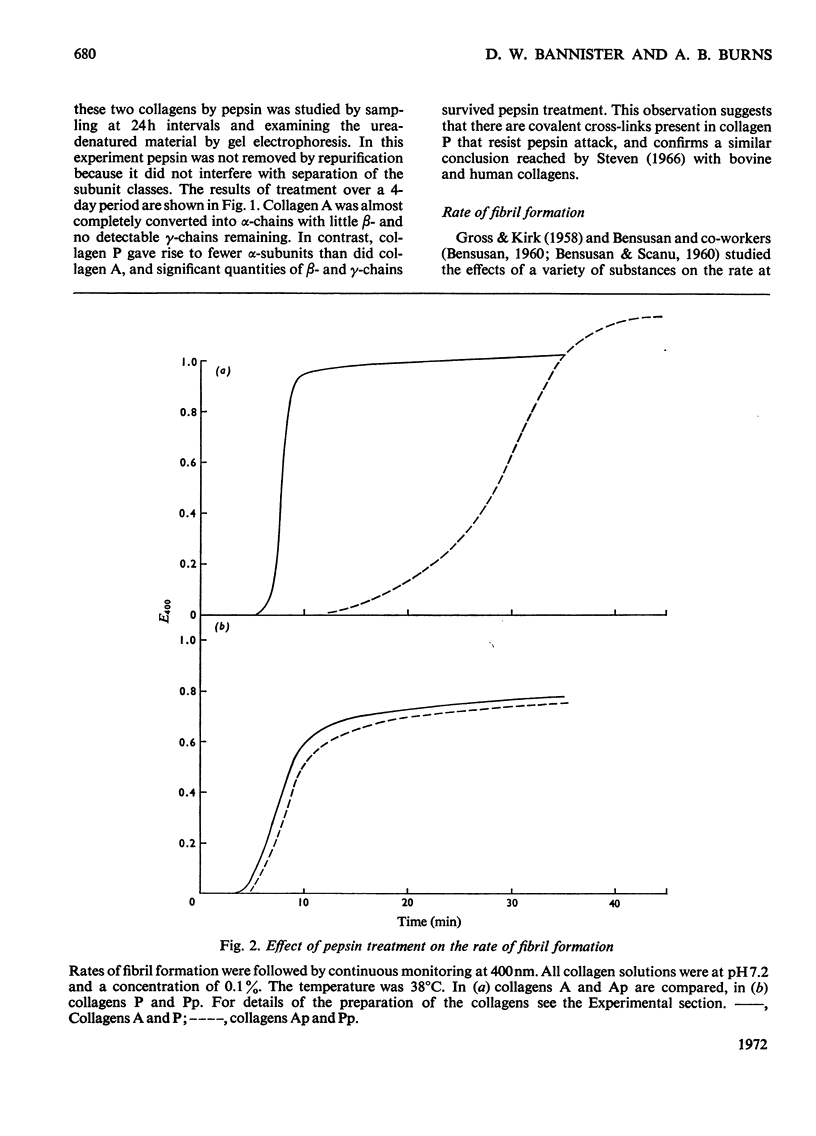
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN P., MARTIN G. R., PIEZ K. A. INTERMOLECULAR CROSS-LINKING OF COLLAGEN AND THE IDENTIFICATION OF A NEW BETA-COMPONENT. Science. 1964 Jun 5;144(3623):1220–1221. doi: 10.1126/science.144.3623.1220. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Nimni M. E. Characterization of the aldehydes present on the cyanogen bromide peptides from mature rat skin collagen. Biochemistry. 1971 Apr 27;10(9):1640–1647. doi: 10.1021/bi00785a022. [DOI] [PubMed] [Google Scholar]
- Fujimoto D. Isolation of collagens of high hydroxyproline, hydroxylsine and carbohydrate content from muscle layer of Ascaris lumbricoides and pig kidney. Biochim Biophys Acta. 1968 Dec 3;168(3):537–543. doi: 10.1016/0005-2795(68)90187-6. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Hodge A. J., Highberger J. H., Deffner G. G., Schmitt F. O. THE EFFECTS OF PROTEASES ON THE TROPOCOLLAGEN MACROMOLECULE AND ON ITS AGGREGATION PROPERTIES. Proc Natl Acad Sci U S A. 1960 Feb;46(2):197–206. doi: 10.1073/pnas.46.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang A. H., Nagai Y., Piez K. A., Gross J. Studies on the structure of collagen utilizing a collagenolytic enzyme from tadpole. Biochemistry. 1966 Feb;5(2):509–515. doi: 10.1021/bi00866a016. [DOI] [PubMed] [Google Scholar]
- LEWIS M. S., PIEZ K. A. SEDIMENTATION-EQUILIBRIUM STUDIES OF THE MOLECULAR WEIGHT OF SINGLE AND DOUBLE CHAINS FROM RAT-SKIN COLLAGEN. Biochemistry. 1964 Aug;3:1126–1131. doi: 10.1021/bi00896a020. [DOI] [PubMed] [Google Scholar]
- LEWIS M. S., PIEZ K. A. THE CHARACTERIZATION OF COLLAGEN FROM THE SKIN OF THE DOGFISH SHARK, SQUALUS ACANTHIAS. J Biol Chem. 1964 Oct;239:3336–3340. [PubMed] [Google Scholar]
- PAZ M. A., BLUMENFELD O. O., ROJKIND M., HENSON E., FURFINE C., GALLOP P. M. DETERMINATION OF CARBONYL COMPOUNDS WITH N-METHYL BENZOTHIAZOLONE HYDRAZONE. Arch Biochem Biophys. 1965 Mar;109:548–559. doi: 10.1016/0003-9861(65)90400-5. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Characterization of a collagen from codfish skin containing three chromatographically different alpha chains. Biochemistry. 1965 Dec;4(12):2590–2596. doi: 10.1021/bi00888a007. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Cross-linking of collagen and elastin. Annu Rev Biochem. 1968;37:547–570. doi: 10.1146/annurev.bi.37.070168.002555. [DOI] [PubMed] [Google Scholar]
- RUBIN A. L., PFAHL D., SPEAKMAN P. T., DAVISON P. F., SCHMITT F. O. Tropocollagen: significance of protease-induced alterations. Science. 1963 Jan 4;139(3549):37–39. doi: 10.1126/science.139.3549.37. [DOI] [PubMed] [Google Scholar]
- STEVEN F. S. THE CLEAVAGE OF TYROSYL PEPTIDES BY PEPSIN FROM COLLAGEN SOLUBILISED BY THE NISHIHARA TECHNIQUE. Biochim Biophys Acta. 1965 Mar 8;97:465–471. doi: 10.1016/0304-4165(65)90158-3. [DOI] [PubMed] [Google Scholar]


