Abstract
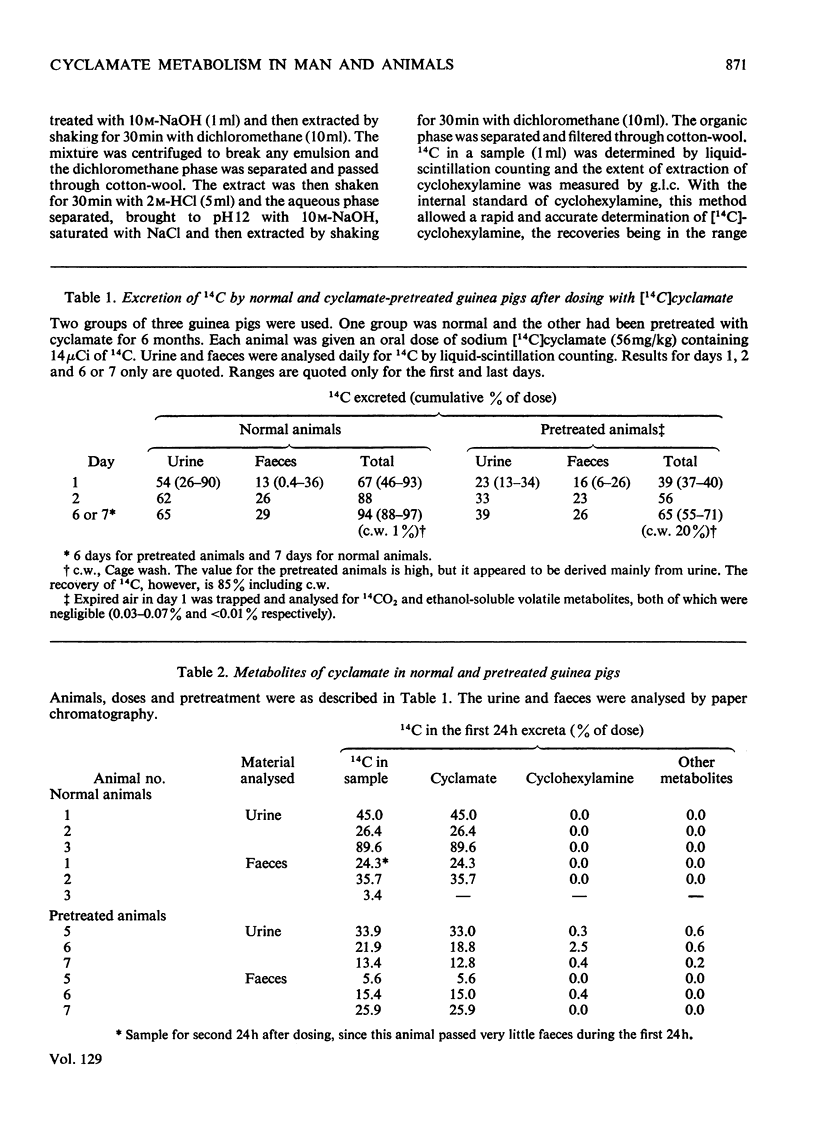
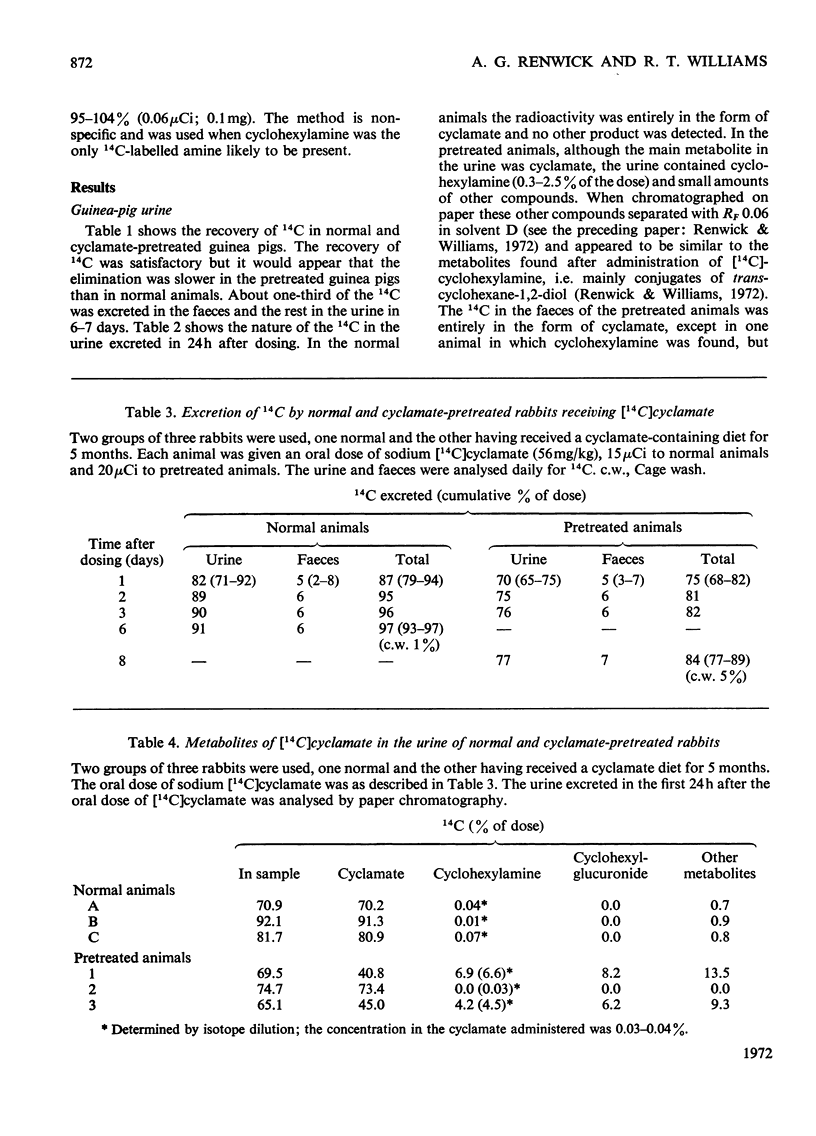
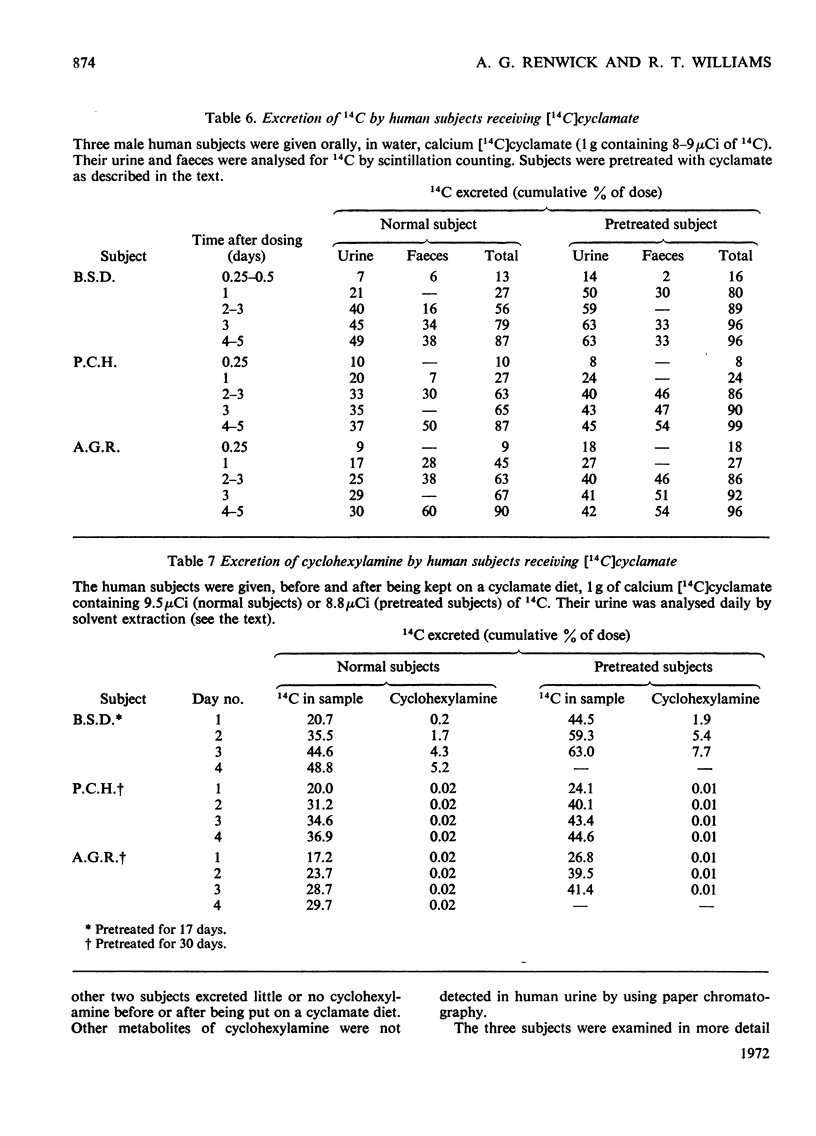
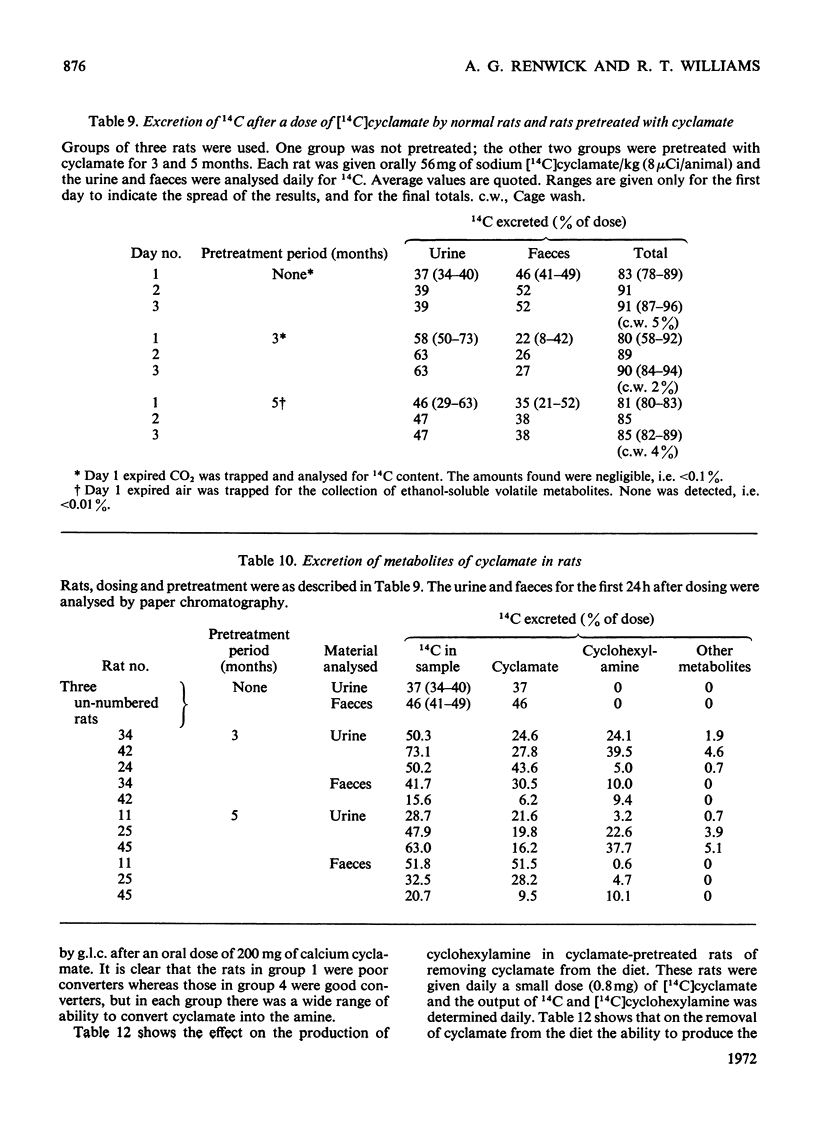
1. 14C-labelled cyclamate has been administered to guinea pigs, rabbits, rats and humans. When given orally to these species on a cyclamate-free diet, cyclamate is excreted unchanged. In guinea pigs some 65% of a single dose is excreted in the urine and 30% in the faeces, the corresponding values for rats being 40 and 50%, for man, 30–50% and 40–60%, and for rabbits, 90 and 5%, the excretion being over a period of 2–3 days. 2. Cyclamate appears to be readily absorbed by rabbits but less readily by guinea pigs, rats and humans. 3. If these animals, including man, are placed on a diet containing cyclamate they develop the ability to convert orally administered cyclamate into cyclohexylamine and consequently into the metabolites of the latter. The extent to which this ability develops is variable, the development occurring more readily in rats than in rabbits or guinea pigs. In three human subjects, one developed the ability quite markedly in 10 days whereas two others did not in 30 days. Removal of the cyclamate from the diet caused a diminution in the ability to convert cyclamate into the amine. 4. In rats that had developed the ability to metabolize orally administered cyclamate, intraperitoneally injected cyclamate was not metabolized and was excreted unchanged in the urine. The biliary excretion of injected cyclamate in rats was very small, i.e. about 0.3% of the dose. 5. The ability of animals to convert cyclamate into cyclohexylamine appears to depend upon a continuous intake of cyclamate and on some factor in the gastrointestinal tract, probably the gut flora.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-El-Makarem M. M., Millburn P., Smith R. L., Williams R. T. Biliary excretion of foreign compounds. Benzene and its derivatives in the rat. Biochem J. 1967 Dec;105(3):1269–1274. doi: 10.1042/bj1051269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina M., Yamaha T., Watanabe K., Sarrazin G. Excretion of cyclohexylamine, a metabolite of cyclamate, in human urine. Chem Pharm Bull (Tokyo) 1971 Mar;19(3):628–632. doi: 10.1248/cpb.19.628. [DOI] [PubMed] [Google Scholar]
- Dalderup L. M., Keller G. H., Schouten F. Cyclamate and cyclohexylamine. Lancet. 1970 Apr 18;1(7651):845–845. doi: 10.1016/s0140-6736(70)92451-7. [DOI] [PubMed] [Google Scholar]
- Davis T. R., Adler N., Opsahl J. C. Excretion of cyclohexylamine in subjects ingesting sodium cyclamate. Toxicol Appl Pharmacol. 1969 Jul;15:106–116. doi: 10.1016/0041-008x(69)90138-0. [DOI] [PubMed] [Google Scholar]
- Drasar B. S., Renwick A. G., Williams R. T. The role of the gut flora in the metabolism of cyclamate. Biochem J. 1972 Oct;129(4):881–890. doi: 10.1042/bj1290881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S., Ichibagase H., Iguchi S. Studies on synthetic sweetening agents. VII. Absorption and excretion of sodium cyclamate. Chem Pharm Bull (Tokyo) 1966 Sep;14(9):965–971. doi: 10.1248/cpb.14.965. [DOI] [PubMed] [Google Scholar]
- Kojima S., Ichibagase H. Studies on synthetic sweetening agents. 13. Metabolism of sodium cyclamate. (2). Detection of metabolites of sodium cyclamate in the rabbit and rat by gas-liquid chromatography. Chem Pharm Bull (Tokyo) 1968 Sep;16(9):1851–1854. doi: 10.1248/cpb.16.1851. [DOI] [PubMed] [Google Scholar]
- Kojima S., Ichibagase H. Studies on synthetic sweetening agents. 8. Cyclohexylamine, a metabolite of sodium cyclamate. Chem Pharm Bull (Tokyo) 1966 Sep;14(9):971–974. doi: 10.1248/cpb.14.971. [DOI] [PubMed] [Google Scholar]
- Kojima S., Ichibagase H. Studies on synthetic sweetening agents. XIV. Metabolism of sodium cyclamate. 3. On metabolites of sodium cyclamate in human. Chem Pharm Bull (Tokyo) 1969 Dec;17(12):2620–2625. doi: 10.1248/cpb.17.2620. [DOI] [PubMed] [Google Scholar]
- Leahy J. S., Taylor T., Rudd C. J. Cyclohexylamine excretors among human volunteers given cyclamate. Food Cosmet Toxicol. 1967 Oct;5(4):595–596. doi: 10.1016/s0015-6264(67)83210-3. [DOI] [PubMed] [Google Scholar]
- Litchfield M. H., Swan A. A. Cyclohexylamine production and physiological measurements in subjects ingesting sodium cyclamate. Toxicol Appl Pharmacol. 1971 Mar;18(3):535–541. doi: 10.1016/s0041-008x(71)80006-6. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Crawford L. E., Sonders R. C., Cardinal E. V. Distribution and excretion of 14C-cyclamate sodium in animals. Biochem Biophys Res Commun. 1966 Oct 20;25(2):153–157. doi: 10.1016/0006-291x(66)90572-9. [DOI] [PubMed] [Google Scholar]
- Oser B. L., Carson S., Vogin E. E., Sonders R. C. Conversion of cyclamate to cyclohexylamine in rats. Nature. 1968 Oct 12;220(5163):178–179. doi: 10.1038/220178a0. [DOI] [PubMed] [Google Scholar]
- Price J. M., Biava C. G., Oser B. L., Vogin E. E., Steinfeld J., Ley H. L. Bladder tumors in rats fed cyclohexylamine or high doses of a mixture of cyclamate and saccharin. Science. 1970 Feb 20;167(3921):1131–1132. doi: 10.1126/science.167.3921.1131. [DOI] [PubMed] [Google Scholar]
- Prosky L., O'Dell R. G. In vivo conversion of 14 C-labeled cyclamate to cyclohexylamine. J Pharm Sci. 1971 Sep;60(9):1341–1343. doi: 10.1002/jps.2600600910. [DOI] [PubMed] [Google Scholar]
- Renwick A. G., Williams R. T. The metabolites of cyclohexylamine in man and certain animals. Biochem J. 1972 Oct;129(4):857–867. doi: 10.1042/bj1290857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR J. D., RICHARDS R. K., DAVIN J. C. Excretion and distribution of radioactive S35-cyclamate sodium (sucaryl sodium) in animals. Proc Soc Exp Biol Med. 1951 Nov;78(2):530–533. doi: 10.3181/00379727-78-19129. [DOI] [PubMed] [Google Scholar]
- Wallace W. C., Lethco E. J., Brouwer E. A. The metabolism of cyclamates in rats. J Pharmacol Exp Ther. 1970 Nov;175(2):325–330. [PubMed] [Google Scholar]


