Abstract
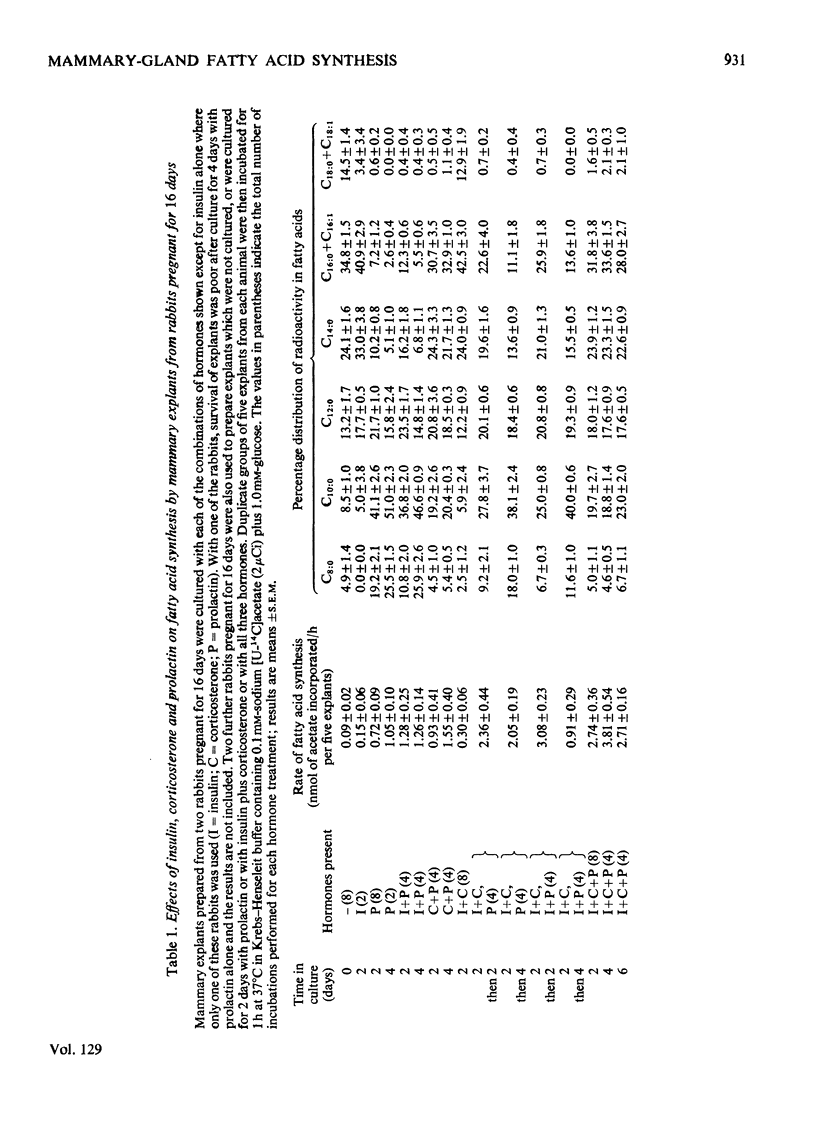
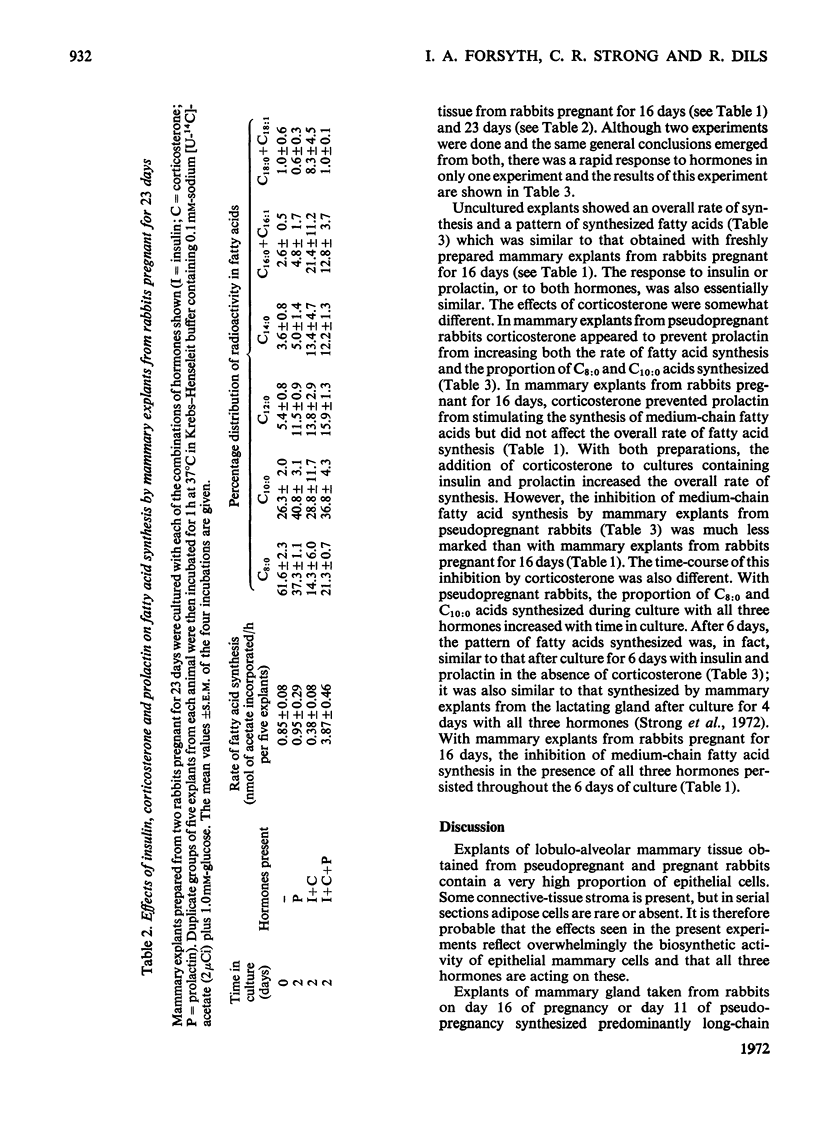
1. The rate of fatty acid synthesis by mammary explants from rabbits pregnant for 16 days or from rabbits pseudopregnant for 11 days was stimulated up to 15-fold by culturing for 2–4 days with prolactin. This treatment initiated the predominant synthesis of C8:0 and C10:0 fatty acids, which are characteristic of rabbit milk. 2. Inclusion of insulin in the culture medium increased the rate of synthesis of these medium-chain fatty acids. By contrast the inclusion of corticosterone led to the predominant synthesis of long-chain fatty acids. When explants were cultured for 2–4 days with insulin, corticosterone and prolactin, the rate of fatty acid synthesis increased up to 42-fold, but both medium- and long-chain fatty acids were synthesized. 3. These results show that the stimulus to mammary-gland lipogenesis and the initiation of synthesis of medium-chain fatty acids observed between days 16 and 23 of pregnancy in the rabbit can be simulated in vitro by prolactin alone. 4. When mammary explants from rabbits pregnant for 23 days were cultured for 2 days with insulin, corticosterone and prolactin, the rate of fatty acid synthesis increased fivefold, but there was a preferential synthesis of long-chain fatty acids. Culture with prolactin alone had little effect on the rate or pattern of fatty acids synthesized. 5. The results are compared with findings in vivo on the control of lipogenesis in the rabbit mammary gland, and are contrasted with the known effects of hormones in vitro on the mammary gland of the mid-pregnant mouse.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton C. E., Bolton A. E. Effect of prolactin on pathways of glucose oxidation in explants from rabbit mammary gland. FEBS Lett. 1970 Sep 6;9(3):177–179. doi: 10.1016/0014-5793(70)80348-9. [DOI] [PubMed] [Google Scholar]
- Carey E. M., Dils R. The pattern of fatty acid synthesis in lactating rabbit mammary gland studied in vivo. Biochem J. 1972 Feb;126(4):1005–1007. doi: 10.1042/bj1261005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick A. Lactogenesis in pseudopregnant rabbits treated with adrenocorticotrophin and adrenal corticosteroids. J Endocrinol. 1971 Jan;49(1):1–8. doi: 10.1677/joe.0.0490001. [DOI] [PubMed] [Google Scholar]
- Cowie A. T., Hartmann P. E., Turvey A. The maintenance of lactation in the rabbit after hypophysectomy. J Endocrinol. 1969 Apr;43(4):651–662. doi: 10.1677/joe.0.0430651. [DOI] [PubMed] [Google Scholar]
- Cowie A. T., Watson S. C. The adrenal cortex and lactogenesis in the rabbit. J Endocrinol. 1966 Jun;35(2):213–214. doi: 10.1677/joe.0.0350213. [DOI] [PubMed] [Google Scholar]
- Delouis C., Denamur R. Induction of lactose synthesis by prolactin in rabbit mammary gland extracts. J Endocrinol. 1972 Feb;52(2):311–319. doi: 10.1677/joe.0.0520311. [DOI] [PubMed] [Google Scholar]
- Denamur R. Reviews of the progress of dairy science. Section A. Physiology. Hormonal control of lactogenesis. J Dairy Res. 1971 Jun;38(2):237–264. doi: 10.1017/s0022029900019348. [DOI] [PubMed] [Google Scholar]
- Falconer I. R., Fiddler T. J. Effects of intraductal administration of prolactin, actinomycin D and cycloheximide on lipoprotein lipase activity in the mammary glands of pseudopregnant rabbits. Biochim Biophys Acta. 1970 Dec 15;218(3):508–514. doi: 10.1016/0005-2760(70)90013-5. [DOI] [PubMed] [Google Scholar]
- Fiddler T. J., Birkinshaw M., Falconer I. R. Effects of intraductal prolactin on some aspects of the ultrastructure and biochemistry of mammary tissue in the pseudopregnant rabbit. J Endocrinol. 1971 Mar;49(3):459–469. doi: 10.1677/joe.0.0490459. [DOI] [PubMed] [Google Scholar]
- Mills E. S., Topper Y. J. Some ultrastructural effects of insulin, hydrocortisone, and prolactin on mammary gland explants. J Cell Biol. 1970 Feb;44(2):310–328. doi: 10.1083/jcb.44.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti R. L., Abraham S. Effects of insulin on glucose metabolism by explants of mouse mammary gland maintained in organ culture. Biochim Biophys Acta. 1966 Aug 24;124(2):280–288. doi: 10.1016/0304-4165(66)90191-7. [DOI] [PubMed] [Google Scholar]
- POPJAK G., HUNTER G. D., FRENCH T. H. Biosynthesis of milk-fat in the rabbit from acetate and glucose; the mode of conversion of carbohydrate into fat. Biochem J. 1953 May;54(2):238–247. doi: 10.1042/bj0540238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter R. E. Feedback effects of thyroxine on the hypothalamus and pituitary of goldfish, Carassius auratus. J Endocrinol. 1971 Sep;51(1):31–39. doi: 10.1677/joe.0.0510031. [DOI] [PubMed] [Google Scholar]
- Smith S., Watts R., Dils R. Quantitative gas-liquid chromatographic analysis of rodent milk triglycerides. J Lipid Res. 1968 Jan;9(1):52–57. [PubMed] [Google Scholar]
- Strong C. R., Forsyth I., Dils R. The effects of hormones on milk-fat synthesis in mammary explants from pseudopregnant rabbits. Biochem J. 1972 Jul;128(3):509–519. doi: 10.1042/bj1280509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALWALKER P. K., NICOLL C. S., MEITES J. Induction of mammary secretion in pregnant rats and rabbits by hydrocortisone acetate. Endocrinology. 1961 Oct;69:802–808. doi: 10.1210/endo-69-4-802. [DOI] [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Wang D. Y., Hallowes R. C., Bealing J., Strong C. R., Dils R. The effect of prolactin and growth hormone on fatty acid synthesis by pregnant mouse mammary gland in organ culture. J Endocrinol. 1972 May;53(2):311–321. doi: 10.1677/joe.0.0530311. [DOI] [PubMed] [Google Scholar]


