Abstract
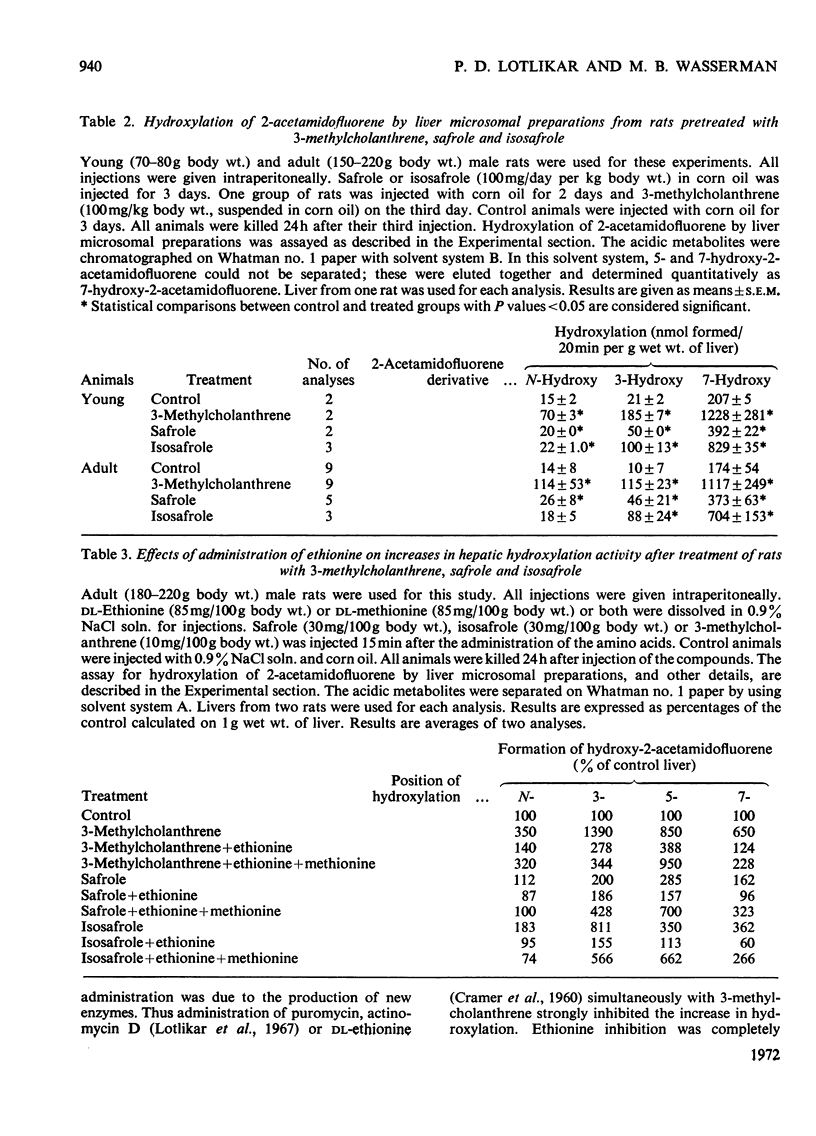
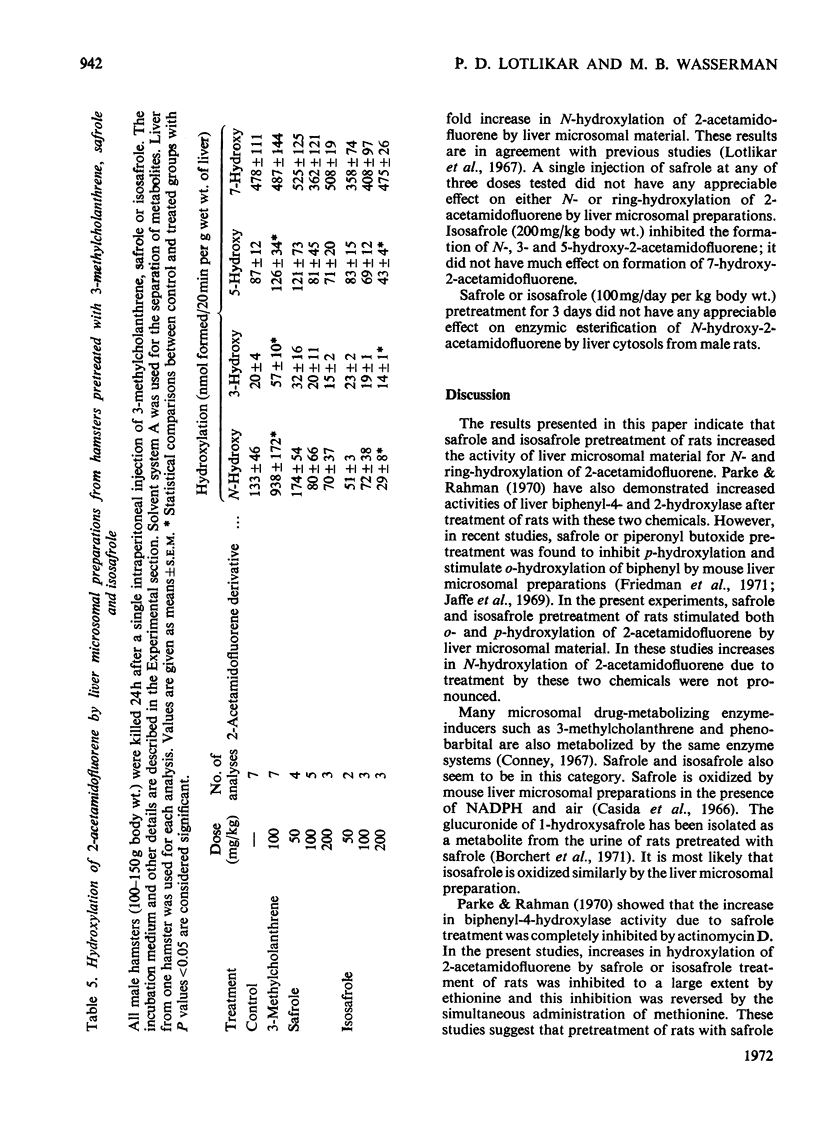
1. The effects of safrole and isosafrole pretreatment on both N- and ring-hydroxylation of 2-acetamidofluorene were studied in male rats and hamsters. 2. Isosafrole (100mg/day per kg body wt.) pretreatment of rats for 3 days did not have any effect on urinary excretion of hydroxy metabolites of 2-acetamidofluorene. However, similar pretreatment with safrole produced increased urinary excretion of N-, 3- and 5-hydroxy derivatives. 3. Similar treatment with these two chemicals for 3 days increased ring-hydroxylation activity by rat liver microsomal material. Increases in N-hydroxylation were much less than those in ring-hydroxylation. Isosafrole was twice as effective as safrole. 4. Increases in hydroxylating activity due to safrole or isosafrole treatment were inhibited by simultaneous administration of ethionine. Similarly, ethionine inhibition was almost completely reversed by the simultaneous administration of methionine. 5. Safrole or isosafrole (0.1mm and 1mm) inhibited 7-hydroxylation activity by liver microsomal material from control rats. At 1mm these two chemicals inhibited both 5- and 7-hydroxylation activity by liver microsomal material from 3-methylcholanthrene-pretreated rats. 3-Hydroxylation activity was not inhibited by 1mm concentrations of these two chemicals. 6. A single injection of safrole (50100 or 200mg/kg body wt.) 24h before assay had no appreciable effect on either N- or ring-hydroxylation activity by hamster liver microsomal material. However, isosafrole (200mg/kg body wt.) treatment inhibited N-, 3- and 5-hydroxylation activities by hamster liver microsomal material; it had no effect on 7-hydroxylation activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOOTH J., BOYLAND E. The biochemistry of aromatic amines. III. Enzymic hydroxylation by rat-liver microsomes. Biochem J. 1957 May;66(1):73–78. doi: 10.1042/bj0660073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAMER J. W., MILLER J. A., MILLER E. C. The hydroxylation of the carcinogen 2-acetylaminofluorene by rat liver: stimulation by pretreatment in vivo with 3-methylcholanthrene. J Biol Chem. 1960 Jan;235:250–256. [PubMed] [Google Scholar]
- Casida J. E., Engel J. L., Essac E. G., Kamienski F. X., Kuwatsuka S. Methylene-C14-dioxyphenyl compounds: metabolism in relation to their synergistic action. Science. 1966 Sep 2;153(3740):1130–1133. doi: 10.1126/science.153.3740.1130. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Enomoto M., Miyake M., Sato K. Carcinogenicity in the hamster of simultaneously administered 2-acetamidofluorene and 3-methylcholanthrene. Gan. 1968 Jun;59(3):177–186. [PubMed] [Google Scholar]
- Epstein S. S., Fujii K., Andrea J., Mantel N. Carcinogenicity testing of selected food additives by parenteral administration to infant Swiss mice. Toxicol Appl Pharmacol. 1970 Mar;16(2):321–334. doi: 10.1016/0041-008x(70)90004-9. [DOI] [PubMed] [Google Scholar]
- Friedman M. A., Arnold E., Bishop Y., Epstein S. S. Additive and synergistic inhibition of mammalian microsomal enzyme functions by piperonyl butoxide, safrole and other methylenedioxyphenyl derivatives. Experientia. 1971 Sep 15;27(9):1052–1054. doi: 10.1007/BF02138875. [DOI] [PubMed] [Google Scholar]
- HOMBURGER F., KELLEY T., Jr, FRIEDLER G., RUSSFIELD A. B. Toxic and possible carcinogenic effects of 4-allyl-1,2-methylenedioxybenzene (Safrole) in rats on deficient diets. Med Exp Int J Exp Med. 1961;4:1–11. doi: 10.1159/000134986. [DOI] [PubMed] [Google Scholar]
- IRVING C. C. ENZYMATIC N-HYDROXYLATION OF THE CARCINOGEN 2-ACETYLAMINOFLUORENE AND THE METABOLISM OF N-HYDROXY-2-ACETYLAMINOFLUORENE-9-14C IN VITRO. J Biol Chem. 1964 May;239:1589–1596. [PubMed] [Google Scholar]
- Jaffe H., Fujii K., Guerin H., Sengupta M., Epstein S. S. Bi-modal effect of piperonyl butoxide on the o- and p-hydroxylations of biphenyl by mouse liver microsomes. Biochem Pharmacol. 1969 May;18(5):1045–1051. doi: 10.1016/0006-2952(69)90108-7. [DOI] [PubMed] [Google Scholar]
- Lotlikar P. D. Effects of 3-methylcholanthrene pretreatment on microsomal hydroxylation of 2-acetamidofluorene by various rat hepatomas. Biochem J. 1970 Jul;118(3):513–518. doi: 10.1042/bj1180513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotlikar P. D. Effects of sex hormones on enzymic esterification of 2-(N-hydroxy-acetamido) fluorene by rat liver cytosol. Biochem J. 1970 Nov;120(2):409–416. doi: 10.1042/bj1200409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotlikar P. D., Enomoto M., Miller J. A., Miller E. C. Species variations in the N- and ring-hydroxylation of 2-acetylaminofluorene and effects of 3-methylcholanthrene pretreatment. Proc Soc Exp Biol Med. 1967 Jun;125(2):341–346. doi: 10.3181/00379727-125-32086. [DOI] [PubMed] [Google Scholar]
- MILLER E. C., MILLER J. A., BROWN R. R., MACDONALD J. C. On the protective action of certain polycyclic aromatic hydrocarbons against carcinogenesis by aminoazo dyes and 2-acetylaminofluorene. Cancer Res. 1958 May;18(4):469–477. [PubMed] [Google Scholar]
- MILLER J. A., CRAMER J. W., MILLER E. C. The N- and ringhydroxylation of 2-acetylaminofluorene during carcinogenesis in the rat. Cancer Res. 1960 Jul;20:950–962. [PubMed] [Google Scholar]
- MIYAJI T., MOSZKOWSKI L. I., SENOO T., OGATA M., ODA T., KAWAI K., SAYAMA Y., ISHIDA H., MATSUO H. [Inhibition of 2-acetylaminofluorene tumors in rats with simultaneously fed 20-methylcholanthrene, 9:10-dimethyl-1:2-benzanthracene and chrysene, and consideration of sex difference in tumor genesis with 2-acetyl-aminoflurene]. Gan. 1953 Sep;44(2-3):281–283. [PubMed] [Google Scholar]
- MORRIS H. P., SOBER H. A., WEISBURGER E. K., WEISBURGER J. H. Chromatographic separation of some metabolites of the carcinogen N-2-fluorenylacetamide. J Natl Cancer Inst. 1956 Sep;17(3):363–374. [PubMed] [Google Scholar]
- Miller J. A. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970 Mar;30(3):559–576. [PubMed] [Google Scholar]
- POIRIER L. A., MILLER J. A., MILLER E. C. The N- and ring-hydroxylation of 2-acetylaminofluorene and the failure to detect N-acetylation of 2-aminofluorene in the dog. Cancer Res. 1963 Jun;23:790–800. [PubMed] [Google Scholar]
- SEAL U. S., GUTMANN H. R. The metabolism of the carcinogen N-(2-fluorenyl) acetamide by liver cell fractions. J Biol Chem. 1959 Mar;234(3):648–654. [PubMed] [Google Scholar]


