Abstract
Cocultures of neurons and astrocytes from the rat striatum were used to determine whether the stimulation of neuronal receptors could affect the level of intercellular communication mediated by gap junctions in astrocytes. The costimulation of N-methyl-D-asparte (NMDA) and muscarinic receptors led to a prominent reduction of astrocyte gap junctional communication (GJC) in coculture. This treatment was not effective in astrocyte cultures, these cells being devoid of NMDA receptors. Both types of receptors contribute synergistically to this inhibitory response, as the reduction in astrocyte GJC was not observed after the blockade of either NMDA or muscarinic receptors. The involvement of a neuronal release of arachidonic acid (AA) in this inhibition was investigated because the costimulation of neuronal NMDA and muscarinic receptors markedly enhanced the release of AA in neuronal cultures and in cocultures. In addition, both the reduction of astrocyte GJC and the release of AA evoked by NMDA and muscarinic receptor costimulation were prevented by mepacrine, a phospholipase A2 inhibitor, and this astrocyte GJC inhibition was mimicked by the exogenous application of AA. Metabolites of AA formed through the cyclooxygenase pathway seem to be responsible for the effects induced by either the costimulation of NMDA and muscarinic neuronal receptors or the application of exogenous AA because, in both cases, astrocyte GJC inhibition was prevented by indomethacin. Altogether, these data provide evidence for a neuronal control of astrocytic communication and open perspectives for the understanding of the modalities through which cholinergic interneurons and glutamatergic inputs affect local circuits in the striatum.
Through their multiple processes, astrocytes are in close association with neurons and play multiple roles in brain functions. Besides their supporting role, these glial cells actively interact with neurons because they sense and respond to neuronal activity and can affect synaptic transmission by releasing chemical transmitters (1, 2). A typical feature of astrocytes is their widespread connection by gap junctions, which allows direct intercellular communication and defines astrocytic networks in culture and brain slice preparations (3). Gap junctions are constituted by clusters of intercellular channels composed of 12-subunit proteins, called connexins, 6 of them corresponding to a hemichannel that belongs to each coupled cell. Astrocytes express several connexins with a predominance for connexin 43 (Cx43), present from embryonic to adult stages, and connexin 30, which appears late in situ and in primary cultures (see refs. 4 and 5). The efficiency of gap junctions in astrocytes can be controlled by several classes of endogenous compounds, including neurotransmitters, neuropeptides, bioactive lipids, and growth factors (see refs. 6 and 7). These regulations, which up to now have only been revealed by exogenous applications of these compounds, suggest that astrocytic networks are subject to remodeling and to some degree of plasticity.
The presence of astrocytes has been shown to enhance the number of synapses and synaptic efficacy (8, 9). Moreover, we have also recently reported that astrocyte gap junctional communication (GJC) is regulated by neuronal activity, indicating that astrocyte gap junctions and Cxs represent targets for neuro–glial interaction (10, 11). A novel concept of neuro–glial interaction involved in brain signaling is thus emerging (see refs. 12 and 13); thanks to their tight interactions, astrocytes control neuronal synaptic transmission, and, reciprocally, neurons regulate astrocyte GJC in astrocytes. A potential modality of interaction between neurons and astrocytes is that the stimulation of neuronal receptors induces the release of products affecting astrocyte GJC. Because cultured astrocytes do not express N-methyl-D-asparte (NMDA) receptors (14), cocultures of neurons and astrocytes provide an appropriate model to address this question by selectively stimulating neurons. Interestingly, in striatal neurons, several biochemical and electrophysiological responses evoked by NMDA are enhanced by acetylcholine (Ach) through the stimulation of muscarinic receptors (15, 16). This could partly account for the modulatory role of cholinergic interneurons on the activity of efferent striatal neurons (see ref. 17). As shown in the present study, when striatal neurons are cocultured with astrocytes, the costimulation of NMDA and muscarinic receptors induces a reduction of astrocyte GJC. Moreover, in this condition, arachidonic acid (AA) is markedly released from striatal neurons. Because AA is a potent inhibitor of astrocyte GJC (18–20), we further investigated whether the inhibitory effect of NMDA and muscarinic receptor costimulation on astrocyte GJC results from the neuronal production of AA.
Materials and Methods
Cell Cultures.
Pregnant rats were killed by prolonged exposure to a high concentration of carbon dioxide. Embryos were removed and placed in PBS supplemented with glucose (33 mM). Striata were dissected from 18-day embryos and mechanically dissociated in PBS/glucose. Primary cultures of neurons (7–8 days in vitro), astrocytes (20–21 days in vitro), and cocultures of neurons and astrocytes were prepared as previously described (10, 21). Cocultures of neurons and astrocytes were used 7 days after the plating of neurons on confluent astrocytes (14 days in vitro). Patch-clamp experiments were performed on spontaneous cocultures (8–12 days in vitro) as previously described (see ref. 10).
Assay for [3H]AA Release.
Primary cultures of either neurons or astrocytes and cocultures of neurons and astrocytes were incubated overnight with 1 μCi/ml [3H]AA (200 Ci/mmol; 1 Ci = 37 GBq) added to the culture medium. After four washes with a Mg2+-free Hepes buffer (in mM: 140 NaCl/5.5 KCl/1.8 CaCl2/10 glucose/10 Hepes with pH fixed at 7.4) containing fatty acid-free BSA (5 mg/ml), cells were exposed to receptor agonists for 15 min in the same medium. When used, mepacrine was added during washing and incubation, and (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK801) and atropine were added 10 min before receptor agonists. At the end of incubation, media were centrifuged (100 × g for 10 min) to remove floating cells, and 3H radioactivity, which, as previously reported (22, 23), consisted of at least 95% [3H]AA, was measured in supernatants.
Determination of GJC.
For pharmacological screening, astrocyte GJC was studied by using the scrape-loading technique (see ref. 10) and quantified by measuring the fluorescence area defined by the spread of Lucifer yellow (LY, 0.1% dilithium salt) by using an image analysis software (NIH IMAGE). Control experiments and pharmacological treatments were performed by incubating either astrocytic cultures or cocultures for 15 min in the Mg2+-free Hepes buffer solution described above. Treatments with mepacrine resulted in autofluorescence of the cells in the same wavelength as LY, thus 0.5% sulforhodamine B was used as the intercellular tracer in these experiments. In all cases, astrocyte GJC was assessed 8 min after scraping by taking five successive photomicrographs per trial with an inverted microscope equipped with appropriate filters. Tested compounds were always present in the washing solutions used after scrape-loading. Quantification of the effects of different treatments on astrocyte GJC was performed by comparing fluorescence areas in control and treated conditions. The percentage of change in GJC was determined by subtracting from the total fluorescence area the first row of cells initially loaded, measured in the presence of the gap junction blocker carbenoxolone (100 μM, 10 min) in separated culture dishes (n = 5).
In addition, another dye-coupling assay was performed by using the patch-clamp technique to load individual astrocytes with LY (0.2%, dipotassium salt) (see ref. 10). After 2 min of whole-cell recording, the pipette was withdrawn, and the microscopic field around the recorded cell was photographed 5 min later under epifluorescence illumination. GJC was quantified by determining the number of adjacent neighboring fluorescent astrocytes (10).
Dye coupling between astrocytes and neurons has been described in young cocultures (24–72 h) (24); however, in the present study we did not detect any heterotypic coupling after 7 days of cocultures with the two techniques described above.
Calcium Imaging.
Measurements of intracellular calcium concentration were made under single emission microfluorimetry by using the cell-permeant fluorescent calcium probe Fluo-4 acetoxymethyl ester. This indicator (3 μM) was loaded into cells by incubation for 1 h at room temperature in the standard Hepes buffer described above. After washing, cells were transferred to the recording chamber of an upright microscope, allowing sequential or continuous perfusion of solutions at a rate of 1 ml/min. Fluo-4 was excited at 488 nm through a 40× water-immersion objective by a monochromator with integrated xenon lamp (75 W). Fluorescent emission of labeled cells at 510 nm was detected with a charge-coupled device camera (Sensicam, PCO). Image acquisition (2–10 Hz) and off-line analysis were processed by using the Axon Imaging WORKBENCH software (Axon Instruments, Foster City, CA). Cells were exposed to NMDA in the presence of tetrodotoxin (0.5 μM) by focal application (30 ms) with a pulse generator (Master 8) connected to a pipette (2-μm tip) placed 50–100 μm away from the targeted cell. Data are expressed as a change in fluorescence over baseline fluorescence (ΔF/F0, %).
Immunoblotting.
Cells were washed with the Hepes buffer solution and lysed with 200 μl of boiled 2% SDS solution containing a mixture of phosphatase inhibitors (1 mM orthovanadate and 10 mM β-glycerophosphate). Protein concentration was determined by the bicinchoninic acid method, using BSA as standard. Samples were separated by electrophoresis on 10% polyacrylamide gels and transferred to nitrocellulose (Hybond-ECL). Blots were incubated with a monoclonal mouse Cx43 antibody (diluted at 1/1,000, Chemicon) followed by horseradish peroxidase-conjugated sheep anti-mouse secondary antibody (diluted at 1/1,000, Amersham Pharmacia), and immunodetection was performed by using a chemiluminescence detection kit (Renaissance kit, NEN). Samples of astrocyte cultures always contained 20 μg of protein. Moreover, coculture samples were collected in a way to contain a similar amount of astrocytic proteins (20 μg), as previously described (10).
Solutions and Chemicals.
All drugs were purchased from Sigma except NMDA and MK801, which were from Tocris Neuramin (Bristol, U.K.), phorbol 12-myristate 13-acetate from Calbiochem, and Fluo-4 from Molecular Probes. With the exception of biochemical assays carried out at 37°C, all experiments were performed at room temperature (20–24°C). NMDA applications were always performed in the presence of D-serine (100 μM) and in the absence of Mg2+.
Statistical Analysis.
Data were expressed as means ± SEM, and n refers to the number of independent experiments. Statistical analysis was performed on raw data, and statistical significance was established at *, P < 0.05 and **, P < 0.01 by appropriate tests as indicated in the figure legends.
Results
NMDA and Muscarinic Neuronal Receptor Costimulation Inhibits Astrocyte GJC in Cocultures.
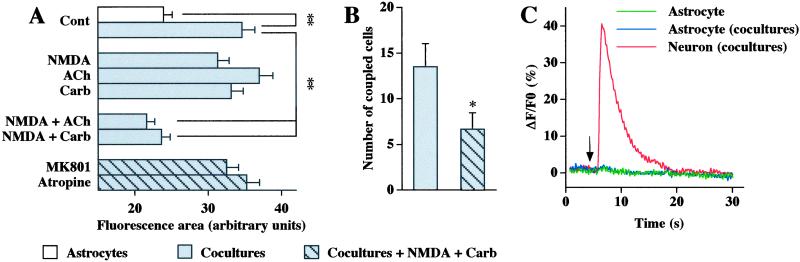
In agreement with previous observations (10), GJC in rat striatal astrocytes was increased in the presence of neurons (Fig. 1A). The intercellular diffusion of LY, studied by using the scrape-loading technique, was not significantly affected when cocultures were exposed to a 15-min treatment with NMDA (100 μM, 0 Mg2+, 100 μM D-serine), ACh (1 mM), or Carb (1 mM) alone (n = 3) (Fig. 1A). In contrast, a 15-min coapplication of NMDA with either ACh or Carb resulted in a significant decrease of astrocyte GJC (44%, n = 5 and 38%, n = 13, respectively). Further indicating the requirement of the stimulation of both receptor types, this inhibition of astrocyte GJC was prevented in the presence of either MK801 (1 μM) or atropine (1 μM), the antagonists of NMDA and muscarinic receptors, respectively (Fig. 1A). The inhibitory effect of NMDA and Carb treatment was confirmed with another dye-coupling assay, which consists of recording a single astrocyte with a patch-clamp pipette filled with LY. Indeed, the occurrence of dye coupling was reduced from 82% (n = 22) to 71% (n = 21), and the average number of coupled cells decreased from 14 ± 3 (n = 18) to 7 ± 2 (n = 15), in controls and treated (NMDA and Carb) cocultures, respectively (Fig. 1B).
Figure 1.
Inhibition of astrocyte dye-coupling by NMDA and muscarinic receptor costimulation in cocultures of neurons and astrocytes. (A) Astrocyte GJC was evaluated in astrocyte cultures and in cocultures of neurons and astrocytes by using the scrape-loading dye transfer technique. The presence of neurons markedly increased astrocyte GJC. No significant change in astrocyte GJC was detected in cocultures incubated during 15 min with NMDA (100 μM, 100 μM D-serine, 0 Mg2+), ACh (1 mM), or carbachol (Carb; 1 mM) alone. In contrast, the fluorescence area was reduced after coapplication of NMDA with either ACh or Carb, and this response was prevented by MK801 (1 μM) or atropine (1 μM). Data, expressed as arbitrary units referring to the fluorescence area defined by the intercellular diffusion of LY, are the means ± SEM of results obtained from 3–32 independent experiments. Statistical significance of treatment effects, when compared with respective control conditions, was determined by one-way ANOVA followed by post hoc Bonferroni's multiple comparison. (B) Dye-coupling experiments performed in astrocytes recorded in cocultures by using the whole-cell configuration with a patch-clamp pipette filled with LY. The number of cells coupled to each recorded astrocyte was significantly reduced in the presence of NMDA and Carb compared with nontreated cocultures. Data are expressed as means ± SEM of results obtained from 18 (control) and 15 (NMDA + Carb) astrocytes, and statistical analysis was carried out by using the Mann–Whitney test. (C) Representative calcium responses obtained on Fluo-4-loaded cells in astrocyte cultures and in cocultures of astrocytes and neurons. Focal application of NMDA, performed in the presence of tetrodotoxin, is indicated by the arrow. An important rise in intracellular calcium was monitored in a neuron cocultured with astrocytes, whereas no detectable change was observed in astrocytes monitored from either astrocyte cultures or cocultures. Similar observations were obtained from 58 astrocytes recorded in the absence of neurons and 62 neurons and 65 astrocytes in cocultures of astrocytes and neurons.
To determine whether the inhibitory effect of the coapplication of NMDA and ACh or Carb resulted from the stimulation of either neuronal or astrocytic receptors, similar experiments were performed on astrocyte cultures. Although muscarinic receptors are expressed in cultured astrocytes from the rat striatum (25), their stimulation by either ACh or Carb did not modify GJC in astrocyte cultures (92% and 87% of the control, respectively, n = 3). In addition, NMDA applied alone or in combination with Carb was also without effect on GJC in astrocyte cultures (102% and 110% of the control, respectively, n = 3). These later results are in agreement with the lack of NMDA receptor expression in cultured astrocytes as indicated by the absence of calcium response to NMDA application (100 μM, 0 Mg2+, 100 μM D-serine) in astrocytes cultured with or without neurons. In contrast, striatal neurons in cocultures exhibited typical rise in intracellular calcium to focal application of NMDA (Fig. 1C).
The Inhibitory Effect of the Costimulation of NMDA and Muscarinic Neuronal Receptors on Astrocyte GJC Is Not Associated with Changes in the Cx43 Expression Pattern.
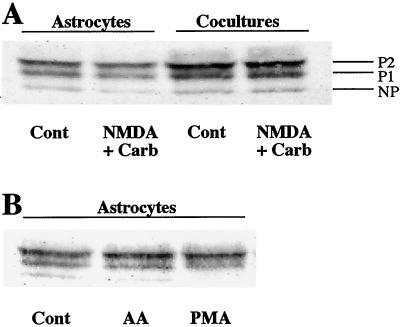
The expression of Cx43 was investigated by immunoblotting to determine whether the decrease in astrocyte GJC induced by the coapplication of NMDA and Carb resulted from a modification in the expression pattern of this major astrocytic connexin (3). Cx43 expression was not detected in pure neuronal cultures (10), but three distinct bands were detected at 42, 44, and 46 kDa in both astrocyte cultures and cocultures (Fig. 2A). These bands correspond to the nonphosphorylated and phosphorylated (P1, P2) isoforms of Cx43, respectively (26). The P2 isoform was predominant in both types of cultures. In agreement with previous observations (10), Cx43 expression was increased without change in its phosphorylation pattern when astrocytes were cocultured for more than 7 days with neurons (Fig. 2A). However, no changes in either the amount or the proportion of these three Cx43 bands were detected (n = 4) after 15 min of coapplication of NMDA and Carb to either the astrocyte cultures or the cocultures (Fig. 2A).
Figure 2.
Western blot analysis of Cx43 expression pattern in astrocyte cultures and in cocultures of neurons and astrocytes. (A) Cx43 expression was enhanced in the presence of neurons after 7 days of coculture, whereas the 15-min coapplication of NMDA (100 μM, 100 μM D-serine, 0 Mg2+) and Carb (1 mM) modified neither the level of expression nor the proportion of Cx43 isoforms in both astrocyte cultures and cocultures. (B) In astrocyte cultures, exogenous AA (5 μM, 15 min) did not modify the distribution pattern of Cx43 isoforms, whereas the nonphosphorylated isoform disappeared after phorbol 12-myristate 13-acetate treatment (PMA; 1 μM, 15 min). Data are representative from three or four independent experiments.
NMDA and Muscarinic Receptor Costimulation Induces the Release of [3H]AA from Neuronal Cultures and Cocultures of Neurons and Astrocytes.
A transcellular messenger such as AA, generated by the stimulation of neuronal receptors, could be responsible for the inhibition of astrocyte GJC induced in cocultures by the coapplication of NMDA and muscarinic receptor agonists. Indeed, according to previous studies, AA is a potent inhibitor of astrocyte GJC (18–20). In addition, as shown in neuronal cultures from the mouse striatum, this unsaturated fatty acid is released after stimulation of NMDA receptors (27), and a synergistic response is observed under costimulation of NMDA and muscarinic receptors (15). Therefore, the evoked AA release was investigated in our different models of rat striatal cultures.
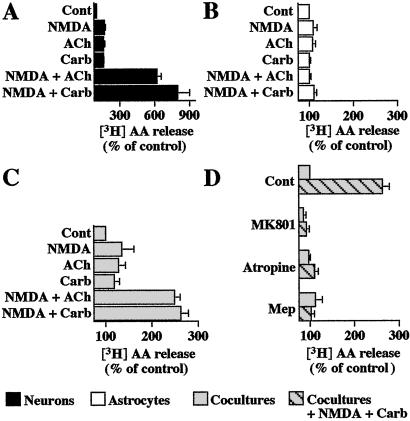
When applied for 15 min, NMDA (100 μM) stimulated [3H]AA release from striatal neuronal cultures prelabeled with [3H]AA (167% of control, n = 4), and similar responses were observed with either ACh (1 mM) or Carb (1 mM) (161%, n = 4 and 159%, n = 3, of control, respectively) (Fig. 3A). A marked synergistic response occurred under the coapplication of NMDA and either ACh or Carb (619%, n = 4 and 800%, n = 3, of control, respectively) (Fig. 3A). These prominent effects were blocked in the presence of mepacrine (50 μM), an inhibitor of phospholipase A2, the main enzyme involved in AA production (data not shown, n = 3). In contrast, in astrocyte cultures, similar concentrations of NMDA, ACh, or Carb had no significant effect on [3H]AA release (n = 3). The lack of effect of these receptor agonists was also observed when ACh or Carb was coapplied with NMDA (Fig. 3B).
Figure 3.
Effects of costimulation of NMDA and muscarinic receptors on AA release. [3H]AA-prelabeled neuronal cultures (A), astrocyte cultures (B), and cocultures (C and D) were incubated for 15 min with NMDA (100 μM, 100 μM D-serine, 0 Mg2+), ACh (1 mM), or Carb (1 mM) applied alone or in combination. The effects of receptor antagonists, MK801 (1 μM), atropine (1 μM), or mepacrine (Mep) (50 μM, 20-min pretreatment), were tested on the response induced by NMDA + Carb in cocultures (D). Values of 3H radioactivity released after stimulation with NMDA + Carb varied from 19,000 to 30,000 dpm per well in neuronal cultures (A) and from 7,000 to 15,000 dpm per well in cocultures (C and D). The reduced capacity of neurons to release [3H]AA in cocultures may be attributed to the presence of serum because the application of 10% serum for 24 h on neuronal cultures reduced by 50% the response evoked by NMDA + Carb costimulation (data not shown). Data are expressed as the means ± SEM of results obtained from at least three independent experiments, each performed in quadruplicate. Statistical analysis was carried out on raw data by one-way ANOVA, followed by post hoc Bonferroni's multiple comparison.
When cocultures were used, NMDA, ACh, or Carb alone significantly evoked [3H]AA release (Fig. 3B). However, the coapplication of NMDA with either ACh or Carb resulted in a marked stimulation of [3H]AA release (249%, n = 4 and 263%, n = 3, of control, respectively) (Fig. 3C). The responses induced by the combined application of NMDA and Carb were completely prevented by MK801 (1 μM), atropine (1 μM), or mepacrine (50 μM) (Fig. 3D). When tested alone on either neuron cultures or cocultures (n = 3), these compounds were without effect.
The Inhibitory Effect of NMDA and Muscarinic Neuronal Receptor Costimulation on Astrocyte GJC Might Operate Through the Production of AA by Neurons.
Because regulations of astrocyte GJC and [3H]AA release exhibited similar pharmacological profiles, we further investigated the possible involvement of AA in the NMDA- and Carb-induced inhibition of astrocyte GJC. For this purpose, several treatments acting on the production, the diffusion, or the metabolism of AA were selected to prevent the effect of neuronal receptor costimulation on astrocyte GJC. In addition, exogenous AA was applied on cocultures to reproduce this astrocyte GJC inhibition, and inhibitors of AA metabolic pathways were used to determine whether it acts directly or through its metabolites.
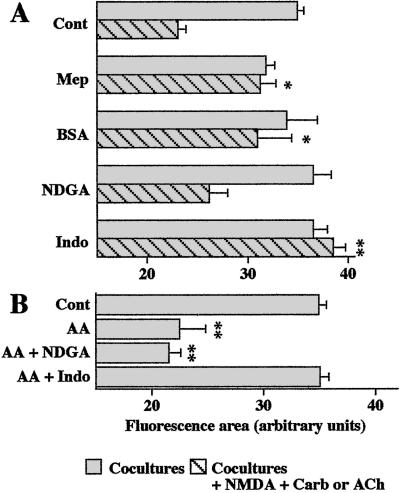
Mepacrine (50 μM) treatment prevented the inhibitory effect of the costimulation of NMDA and muscarinic receptors on astrocyte GJC in cocultures. This inhibition of astrocyte GJC was also abolished when experiments were performed in the presence of BSA (5 mg/ml), known to possess hydrophobic sites that trap fatty acids (Fig. 4A). When used alone, either mepacrine or BSA was without effect on GJC (Fig. 4A). Altogether, these observations are consistent with the involvement of AA release from neurons in the reduction of astrocyte GJC induced by the costimulation of NMDA and muscarinic receptors.
Figure 4.
Involvement of AA in the inhibition of astrocyte GJC induced by costimulation of neuronal NMDA and muscarinic receptors studied by scrape-loading in cocultures. (A) The astrocyte uncoupling induced by NMDA + Carb or NMDA + ACh (100 μM and 1 mM, respectively, 15 min) was reversed by treatment of the cocultures with mepacrine (Mep; 50 μM, 20-min pretreatment), BSA (5 mg/ml), or indomethacin (Indo; 10 μM, 30-min pretreatment), but not by nordihydroguaiaretic acid (NDGA; 5 μM, 30-min pretreatment). (B) Effects of either NDGA or indomethacin on the inhibition of astrocyte GJC induced by exogenous AA (5 μM, 15 min) in cocultures. Analysis were carried out from 3 to 25 independent experiments. Statistical significance of treatments, when compared to the effect of NMDA + Carb or ACh (A) or to control (B), were established by one-way ANOVA followed by post hoc Dunnett's multiple comparison.
In astrocytes, AA is metabolized mainly through the lipoxygenase and cyclooxygenase pathways (22). Therefore, cocultures were preincubated with either nordihydroguaiaretic acid (NDGA; 5 μM, 30 min), a lipoxygenase inhibitor, or indomethacin (10 μM, 30 min), a cyclooxygenase inhibitor, to determine whether AA itself and/or its metabolites are responsible for the decrease of astrocyte GJC induced by the costimulation of NMDA and muscarinic receptors. Whereas NDGA was ineffective, indomethacin pretreatment completely prevented the inhibition of astrocyte GJC (Fig. 4A). When used alone, both inhibitors were without effect on astrocyte GJC (n = 3) (data not shown).
Finally, experiments were performed with exogenous AA to further investigate whether this fatty acid is involved in the reduction of astrocyte GJC observed after the 15-min coapplication of NMDA and Carb (40%, n = 18). As shown in Fig. 4B, when applied for 15 min at 5 μM to the cocultures, AA reduced to a similar extent astrocyte GJC (42%, n = 4). In agreement with previous results (20), this inhibitory effect of AA on astrocyte GJC was not associated with a change in Cx43 expression and phosphorylation, which is in contrast with that found with the phorbol ester (1 μM phorbol 12-myristate 13-acetate, 15 min), another agent known to inhibit astrocyte GJC (Fig. 2B). Moreover, as observed under the combined stimulation of NMDA and muscarinic receptors, the inhibition of astrocyte GJC induced by the exogenous application of AA was prevented by pretreatment of the cocultures with indomethacin, but not NDGA (Fig. 4B).
Discussion
The main finding of this study is that neuronal receptor stimulation can be associated with a change in intercellular communication between astrocytes. Indeed, the costimulation of NMDA and muscarinic receptors in cocultures was found to decrease astrocyte GJC. This observation reinforces the concept of tight neuro–glial interaction (2, 13) and of mutual control of communication between these two cell types (10–12). That neuronal receptor stimulation is indeed involved in this response is based mainly on the presence of NMDA receptors on neurons, but not on astrocytes (Fig. 1C). In addition, although muscarinic receptors are expressed in cultures of striatal astrocytes (25), the synergistic effects of NMDA and either ACh or Carb on AA release were detected only in neuronal cultures and in cocultures. Similarly, the inhibitory effect of the costimulation of NMDA and muscarinic receptors on astrocyte GJC was observed in cocultures but not in astrocyte cultures. These observations suggest that the interaction between the two receptor pathways occurs in neurons. The reduction of astrocyte GJC induced by a 15-min coapplication of NMDA and muscarinic receptor agonists suggests a rapid and tight interaction between neurons and astrocytes, which are closely associated in several brain structures (9, 28, 29).
Several mechanisms that could account for the inhibition of astrocyte GJC by NMDA and muscarinic receptor costimulation can be excluded. (i) Release of glutamate and γ-aminobutyric acid (GABA) by striatal neurons is not involved in this inhibition, as glutamate was reported to increase GJC, and GABA has no effect on astrocyte GJC (10); (ii) direct communication through gap junctions between astrocytes and neurons is also excluded because heterotypic dye coupling is not detected in 1-week-old cocultures; and (iii) an NMDA neurotoxicity is unlikely to occur because longer time of NMDA exposure is required to observe striatal neuronal death (30). Moreover, in striatal cocultures NMDA-induced neurotoxicity is associated with a down-regulation of GJC and Cx43 expression (10), which was not observed in the present study (Fig. 3). Alternatively, the neuro–glial interaction leading to the inhibition of astrocyte GJC is more likely because of the release by neurons of a factor that acts either directly or indirectly on astrocyte GJC. Because of its amphipathic properties, AA is a good candidate to act as a transcellular messenger (see ref. 31). Indeed, several observations support the involvement of AA released from neurons in the reduction of astrocyte GJC induced by the costimulation of NMDA and muscarinic receptors. (i) Although changes in AA release and astrocyte GJC could not be detected in cocultures under the application of NMDA, ACh, or Carb alone, a marked release of AA and reduction of astrocyte GJC were observed under the coapplication of NMDA with either ACh or Carb, and both responses were blocked by either MK801 or atropine; (ii) both the release of AA and the reduction of astrocyte GJC induced by the costimulation of NMDA and muscarinic receptors were prevented when cocultures were pretreated with mepacrine, an inhibitor of phospholipase A2; (iii) the inhibitory effect of the NMDA and muscarinic receptor costimulation on astrocyte GJC was not observed anymore when AA released from neurons was extracellularly trapped with BSA; (iv) the application of 5 μM AA reduced astrocyte GJC to a similar extent as the coapplication of NMDA and Carb (or ACh); and (v) the level and pattern of Cx43 expression was not affected by the application of either AA or NMDA and Carb.
Therefore, a sequence of intracellular and intercellular events leading to the release of AA seems to be involved in the neuronal inhibitory regulation of GJC in astrocytes (Fig. 5). AA metabolites seem to be responsible for the reduction of astrocyte GJC because the inhibitory effects of AA, either exogenous or endogenously formed (costimulation of NMDA and muscarinic receptors), on astrocyte GJC were totally prevented when cocultures were pretreated with indomethacin. Such a critical role of the cyclooxygenase metabolic pathway has already been reported for the inhibitory effect of exogenous AA on astrocyte GJC as well as for the reoxygenation-induced uncoupling of astrocytes (20, 32). Further indicating that cyclooxygenases contribute indeed to neuro–glial interaction, this metabolic pathway was reported to be involved in neuronal responses induced by a calcium-dependent release of glutamate from neighboring astrocytes (33).
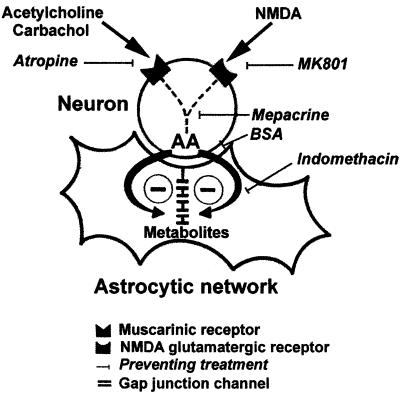
Figure 5.
Summary diagram illustrating the putative mechanism of astrocyte GJC inhibition induced by neuronal receptor costimulation in striatal cocultures. The costimulation of neuronal muscarinic and NMDA glutamatergic receptors induces the release of AA from neurons. AA acts as a transcellular messenger, and its metabolites produced through the cyclooxygenase pathway inhibit GJC in astrocytic networks. Treatments, blocking either NMDA or muscarinic neuronal receptors (atropine and MK801, respectively), or preventing the production (mepacrine), the diffusion (BSA), or the metabolism of AA through the cyclooxygenase pathway (indomethacin), suppress this neuronal modulation of astrocyte GJC.
The medium-sized spiny GABAergic efferent neurons represent more than 95% of the neuronal cell population in the striatum. These neurons are also predominant in neuronal cultures (34) and in cocultures (10) originating from the embryonic striatum. In situ, the dendrites of these striatal neurons are massively innervated by glutamatergic fibers from cortical and thalamic neurons and express several subclasses of glutamatergic receptors, including NMDA receptors. Moreover, the striatum is homogeneously innervated by cholinergic interneurons, which also contact the dendrites of efferent GABAergic neurons (see refs. 17 and 35). Therefore, the costimulation of glutamatergic and cholinergic receptors, including NMDA and muscarinic receptors, may induce specific electrical and biochemical responses in striatal efferent neurons. For instance, the stimulation of muscarinic receptor increases NMDA-dependent currents in striatal slices (16, 17). Thus, in addition to its direct effect on membrane excitability of medium spiny neurons, ACh affects the activity of these cells by interfering with their response to NMDA receptor stimulation (see ref. 17). Interactions between glutamatergic and cholinergic systems may also result from alternative pathways leading to the production of a transcellular messenger and its contribution to neuro–glial interaction. The present results indicate that AA release resulting from the costimulation of neuronal NMDA and muscarinic receptors induces the inhibition of astrocyte GJC. This reduction in the extent of the astrocytic network could secondarily interfere with neuronal activity. Indeed, astrocyte GJC contributes to the propagation of intercellular calcium waves in astrocytes (see refs. 36 and 37) and rises in intracellular calcium in astrocytes modulate synaptic transmission (1). Because increasing evidence supports the occurrence of a tight and active dialogue between neurons and astrocytes (2, 13, 38), the inhibition of astrocyte GJC by neuronal receptor costimulation could contribute to such an integrative process. Accordingly, a reduction in the number of coupled astrocytes could minimize astrocyte–neuron interactions and thus contribute to the role of striatal cholinergic interneurons in the processing of glutamatergic cortical inputs.
Acknowledgments
We thank Dr. A. Koulakoff and L. Venance for helpful discussion and their comments on the manuscript. This work was supported by European Community Grant QLK6-1999-02203.
Abbreviations
- NMDA
N-methyl-d-aspartate
- GJC
gap junctional communication
- AA
arachidonic acid
- Cx43
connexin 43
- LY
Lucifer yellow
- MK801
(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate
- ACh
acetylcholine
- Carb
carbachol
- NDGA
nordihydroguaiaretic acid
- GABA
γ-aminobutyric acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Araque A, Parpura V, Sanzgiri R-P, Haydon P-G. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 2.Haydon P-G. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 3.Rozental R, Giaume C, Spray D-C. Brain Res Rev. 2000;32:11–15. doi: 10.1016/s0165-0173(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 4.Dermietzel R, Spray D-C. Glia. 1998;24:1–7. doi: 10.1002/(sici)1098-1136(199809)24:1<1::aid-glia1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Nagy J-I, Rash J-E. Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 6.Giaume C, McCarthy K-D. Trends Neurosci. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- 7.Reuss B, Dermietzel R, Unsicker K. Glia. 1998;22:19–30. [PubMed] [Google Scholar]
- 8.Pfrieger F-W, Barres B-A. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 9.Ullian E-M, Sapperstein S-K, Christopherson K-S, Barres B-A. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 10.Rouach N, Glowinski J, Giaume C. J Cell Biol. 2000;149:1513–1526. doi: 10.1083/jcb.149.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouach N, Giaume C. In: Progress in Brain Research. Castellano Lopez B, Nieto-Sampedro M, editors. Amsterdam: Elsevier; 2001. pp. 213–224. [Google Scholar]
- 12.Haydon P-G. Curr Biol. 2000;10:R712–R714. doi: 10.1016/s0960-9822(00)00708-9. [DOI] [PubMed] [Google Scholar]
- 13.LoTurco J-J. Proc Natl Acad Sci USA. 2000;97:8196–8197. doi: 10.1073/pnas.97.15.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkhratsky A, Steinhauser C. Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 15.Tencé M, Murphy N, Cordier J, Prémont J, Glowinski J. J Neurochem. 1995;64:1605–1613. doi: 10.1046/j.1471-4159.1995.64041605.x. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Eur J Neurosci. 1998;10:2887–2895. doi: 10.1111/j.1460-9568.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 17.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 18.Giaume C, Marin P, Cordier J, Prémont J. Proc Natl Acad Sci USA. 1991;88:5577–5581. doi: 10.1073/pnas.88.13.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavado E, Sanchez-Abarca L-I, Tabernero A, Bolanos J-P, Medina J-M. J Neurochem. 1997;69:721–728. doi: 10.1046/j.1471-4159.1997.69020721.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez A-D, Saez J-C. Brain Res. 1999;816:411–423. doi: 10.1016/s0006-8993(98)01016-6. [DOI] [PubMed] [Google Scholar]
- 21.El-Etr M, Cordier J, Glowinski J, Prémont J. J Neurosci. 1989;9:1473–1480. doi: 10.1523/JNEUROSCI.09-05-01473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oomagari K, Buisson B, Dumuis A, Bockaert J, Pin J-P. Eur J Neurosci. 1991;3:928–939. doi: 10.1111/j.1460-9568.1991.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 23.Tencé M, Cordier J, Glowinski J, Prémont J. Eur J Neurosci. 1992;4:993–999. doi: 10.1111/j.1460-9568.1992.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 24.Froes M M, Correia A H, Garcia-Abreu J, Spray D C, Campos de Carvalho A C, Neto M V. Proc Natl Acad Sci USA. 1999;96:7541–7546. doi: 10.1073/pnas.96.13.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venance L, Prémont J, Glowinski J, Giaume C. J Physiol. 1998;510:429–440. doi: 10.1111/j.1469-7793.1998.429bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musil L-S, Cunningham B-A, Edelman G-M, Goodenough D-A. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumuis A, Sebben M, Haynes L, Pin J-P, Bockaert J. Nature (London) 1988;336:68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- 28.Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 29.Ventura R, Harris K-M. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maus M, Marin P, Israel M, Glowinski J, Prémont J. Eur J Neurosci. 1999;11:3215–3224. doi: 10.1046/j.1460-9568.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- 31.Axelrod J. Trends Neurosci. 1995;18:64–65. doi: 10.1016/0166-2236(95)93873-v. [DOI] [PubMed] [Google Scholar]
- 32.Martinez A-D, Saez J-C. Brain Res Rev. 2000;32:250–258. doi: 10.1016/s0165-0173(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 33.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini B-L, Pozzan T, Volterra A. Nature (London) 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 34.Fraser D-D, Hoehn K, Weiss S, MacVicar B-A. Neuron. 1993;11:633–644. doi: 10.1016/0896-6273(93)90075-3. [DOI] [PubMed] [Google Scholar]
- 35.Graybiel A-M, Aosaki T, Flaherty A-W, Kimura M. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 36.Giaume C, Venance L. Glia. 1998;24:50–61. [PubMed] [Google Scholar]
- 37.Charles A. Glia. 1998;24:39–49. doi: 10.1002/(sici)1098-1136(199809)24:1<39::aid-glia5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 38.Carmignoto G. Prog Neurobiol. 2000;62:561–581. doi: 10.1016/s0301-0082(00)00029-0. [DOI] [PubMed] [Google Scholar]