Abstract
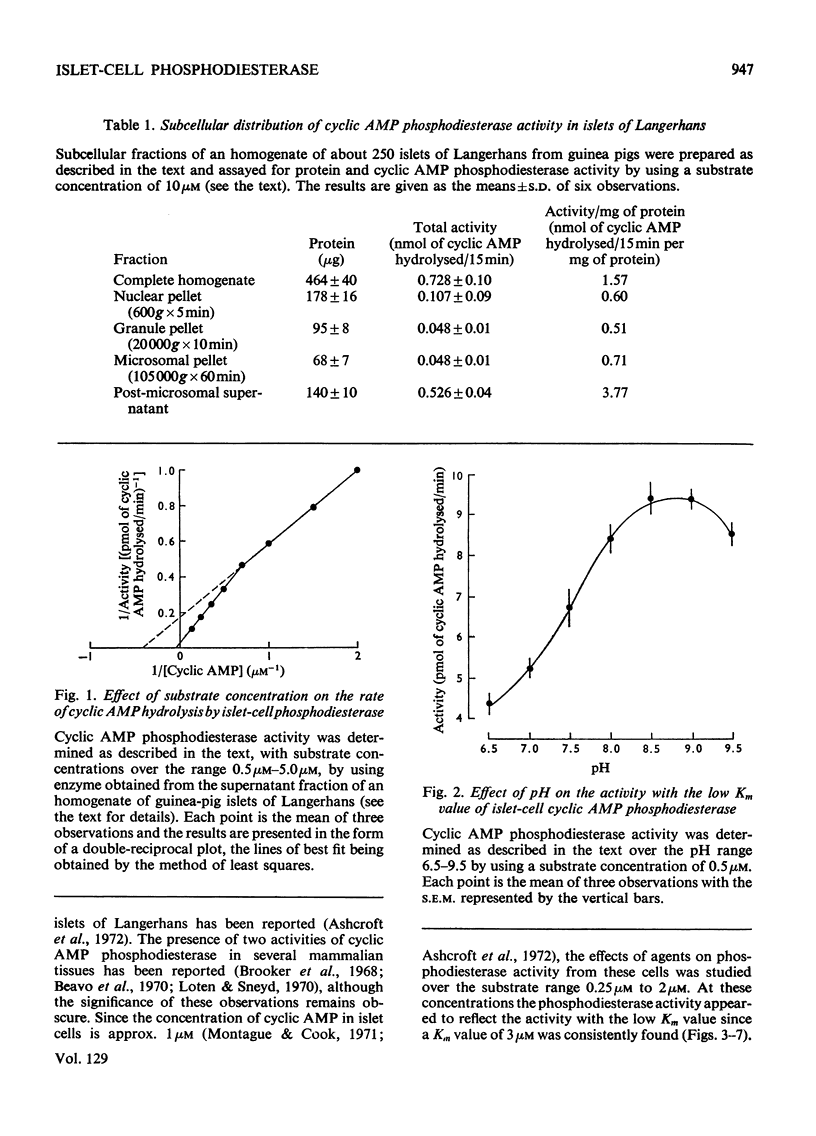
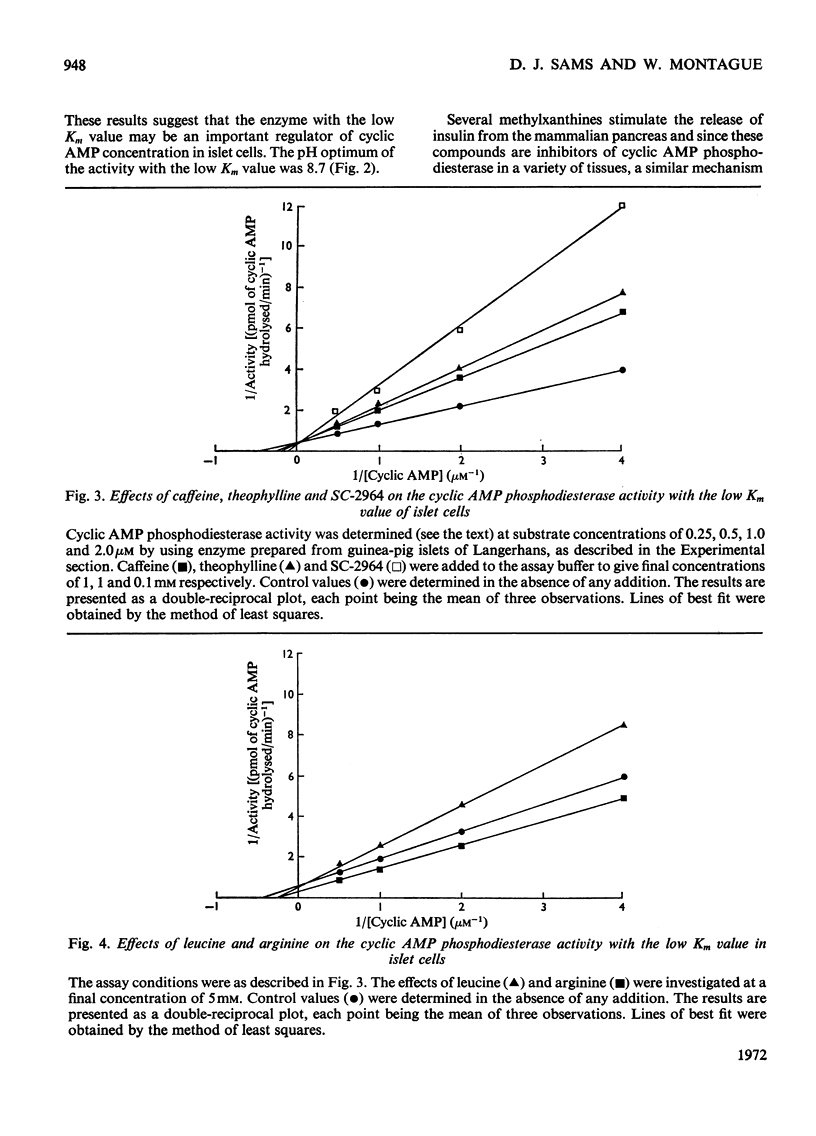
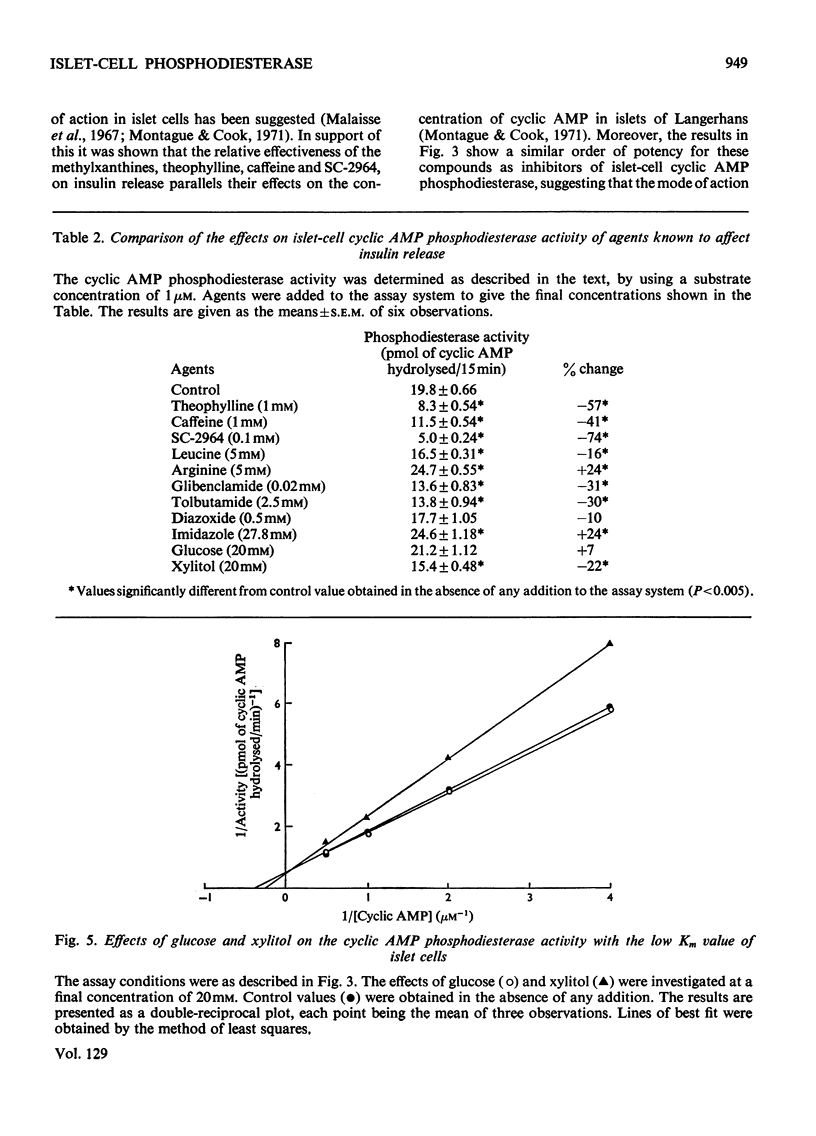
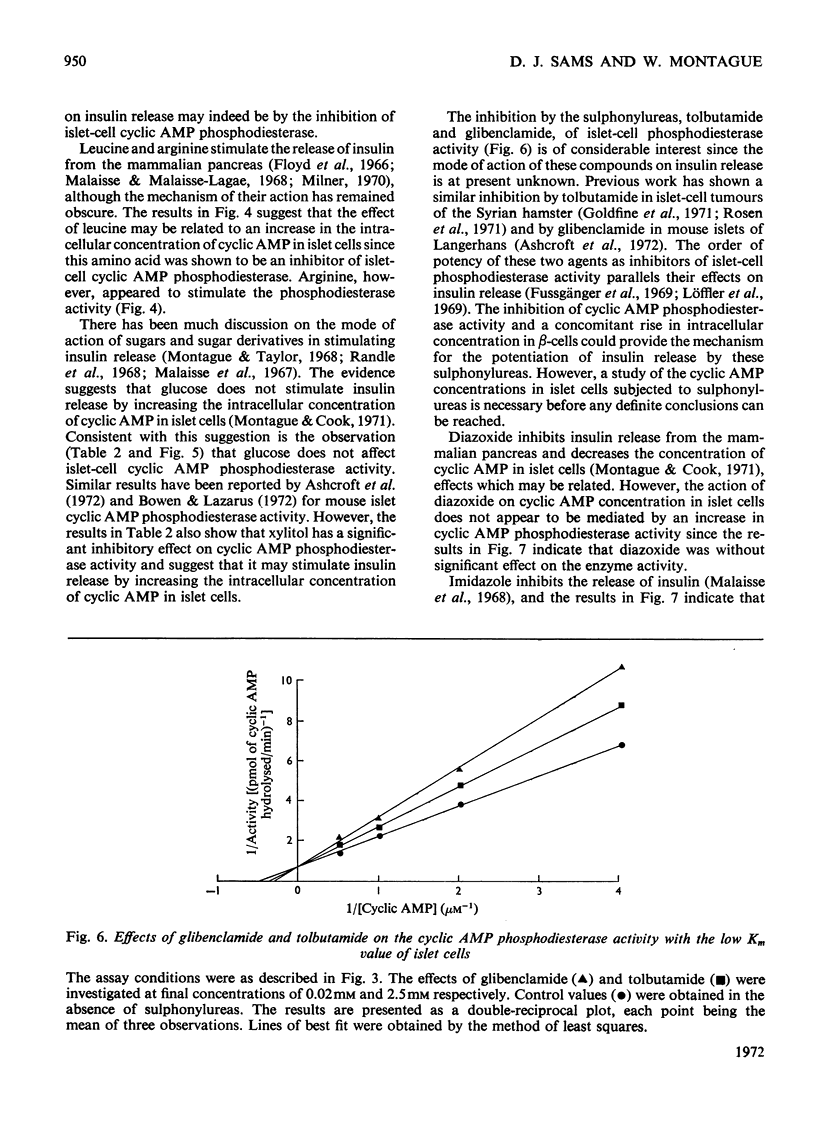
1. An assay has been developed with sufficient sensitivity for determination of the adenosine 3′:5′-cyclic monophosphate diesterase activity in islets of Langerhans, and has been used to investigate the response of the enzyme to various agents which are known to affect insulin release. 2. The subcellular distribution of the enzyme in islets of Langerhans prepared from guinea-pig pancreas was investigated and over 70% of the activity present in the original homogenate was recovered in the supernatant fraction. 3. Gel filtration of the activity present in the supernatant fraction on Sephadex G-200 gave a single peak of activity with an apparent molecular weight of 200000. The phosphodiesterase activity in the peak fraction showed two apparent Km values for adenosine 3′:5′-cyclic monophosphate (cyclic AMP) of 3μm and 30μm, suggesting the presence of two activities. The pH optimum of the activity with the low Km value was 8.7. 4. Theophylline, caffeine, 3-isobutyl-1-methylxanthine (SC-2964), glibenclamide, tolbutamide, xylitol and leucine were inhibitors of the activity with the low Km value; imidazole and arginine stimulated the activity, and glucose and diazoxide were without significant effect. 5. It is suggested that the agents theophylline, caffeine, SC-2964, glibenclamide, tolbutamide, leucine and imidazole may alter the intracellular concentration of cyclic AMP in islets of Langerhans by affecting the cyclic AMP phosphodiesterase activity in islet cells and in this way may affect insulin release.
Full text
PDF

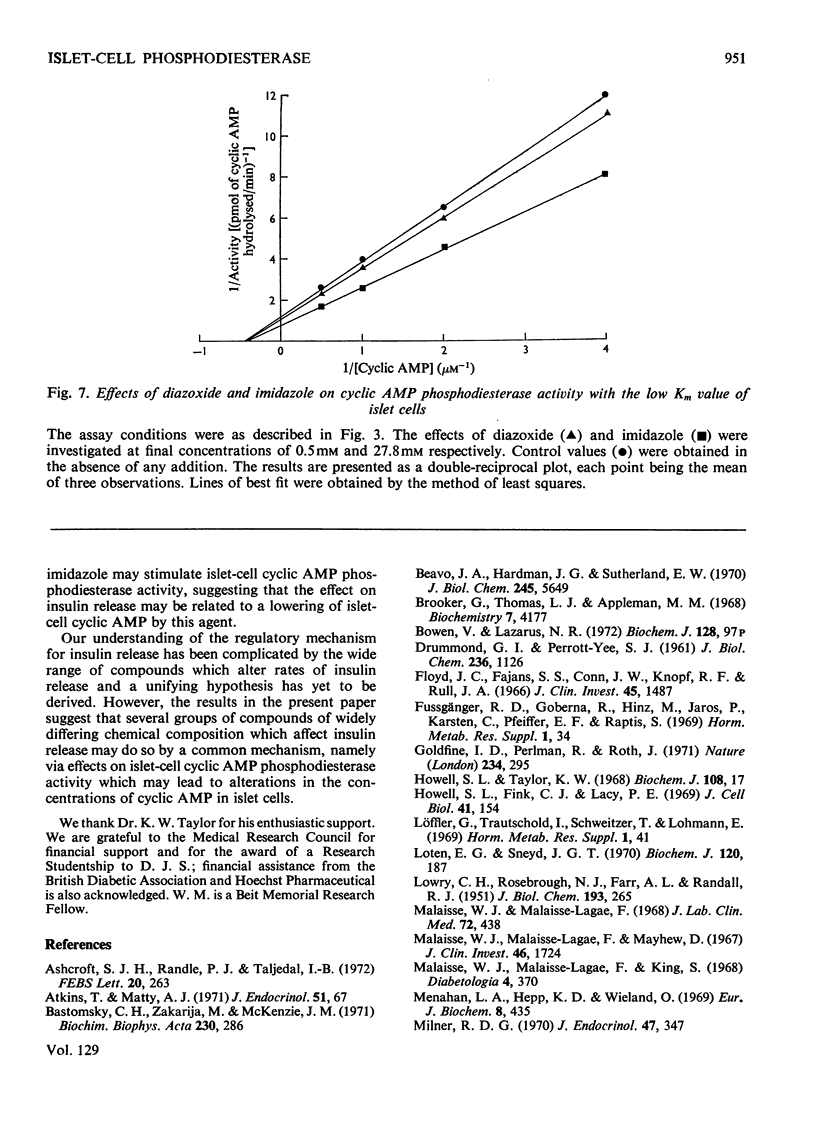

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J.H., Randle P. J., Täljedal I. -B. Cyclic nucleotide phosphodiesterase activity in normal mouse pancreatic islets. FEBS Lett. 1972 Feb 15;20(3):263–266. doi: 10.1016/0014-5793(72)80082-6. [DOI] [PubMed] [Google Scholar]
- Atkins T., Matty A. J. [Six years' experience with a musculoplastic technic]. J Endocrinol. 1971 Sep;51(1):67–78. doi: 10.1677/joe.0.0510067. [DOI] [PubMed] [Google Scholar]
- Bastomsky C. H., Zakarija M., McKenzie J. M. Thyroid hydrolysis of cyclic AMP as influenced by thyroid gland activity. Biochim Biophys Acta. 1971 Feb 23;230(2):286–295. doi: 10.1016/0304-4165(71)90215-7. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Hydrolysis of cyclic guanosine and adenosine 3',5'-monophosphates by rat and bovine tissues. J Biol Chem. 1970 Nov 10;245(21):5649–5655. [PubMed] [Google Scholar]
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- DRUMMOND G. I., PERROTT-YEE S. Enzymatic hydrolysis of adenosine 3',5'-phosphoric acid. J Biol Chem. 1961 Apr;236:1126–1129. [PubMed] [Google Scholar]
- Floyd J. C., Jr, Fajans S. S., Conn J. W., Knopf R. F., Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966 Sep;45(9):1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Perlman R., Roth J. Inhibition of cyclic 3',5'-AMP phosphodiesterase in islet cells and other tissues by tolbutamide. Nature. 1971 Dec 3;234(5327):295–297. doi: 10.1038/234295a0. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Fink C. J., Lacy P. E. Isolation and properties of secretory granules from rat islets of Langerhans. I. Isolation of a secretory granule fraction. J Cell Biol. 1969 Apr;41(1):154–161. doi: 10.1083/jcb.41.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J. 1968 Jun;108(1):17–24. doi: 10.1042/bj1080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loten E. G., Sneyd J. G. An effect of insulin on adipose-tissue adenosine 3':5'-cyclic monophosphate phosphodiesterase. Biochem J. 1970 Nov;120(1):187–193. doi: 10.1042/bj1200187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. Stimulation of insulin secretion by noncarbohydrate metabolites. J Lab Clin Med. 1968 Sep;72(3):438–448. [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., King S. Effects of neutral red and imidazole upon insulin secretion. Diabetologia. 1968 Dec;4(6):370–374. doi: 10.1007/BF01211774. [DOI] [PubMed] [Google Scholar]
- Menahan L. A., Hepp K. D., Wieland O. Liver 3':5'-nucleotide phosphodiesterase and its activity in rat livers perfused with insulin. Eur J Biochem. 1969 Apr;8(3):435–443. doi: 10.1111/j.1432-1033.1969.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Milner R. D. The stimulation of insulin release by essential amino acids from rabbit pancreas in vitro. J Endocrinol. 1970 Jul;47(3):347–356. doi: 10.1677/joe.0.0470347. [DOI] [PubMed] [Google Scholar]
- Montague W., Cook J. R. The role of adenosine 3':5'-cyclic monophosphate in the regulation of insulin release by isolated rat islets of Langerhans. Biochem J. 1971 Mar;122(1):115–120. doi: 10.1042/bj1220115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem J. 1968 Sep;109(3):333–339. doi: 10.1042/bj1090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Hirsch A. H., Goren E. N. Factors which influence cyclic AMP formation and degradation in an islet cell tumor of the Syrian hamster. Arch Biochem Biophys. 1971 Oct;146(2):660–663. doi: 10.1016/0003-9861(71)90175-5. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Vaughan G. D. Insulin release after ACTH, glucagon and adenosine-3'-5'-phosphate (cyclic AMP) in the perfused isolated rat pancreas. Diabetes. 1967 Jul;16(7):449–454. doi: 10.2337/diab.16.7.449. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. An adrenergic receptor mechanism for the control of cyclic 3'5' adenosine monophosphate synthesis in tissues. Biochem Biophys Res Commun. 1967 Sep 7;28(5):797–802. doi: 10.1016/0006-291x(67)90388-9. [DOI] [PubMed] [Google Scholar]


