Abstract
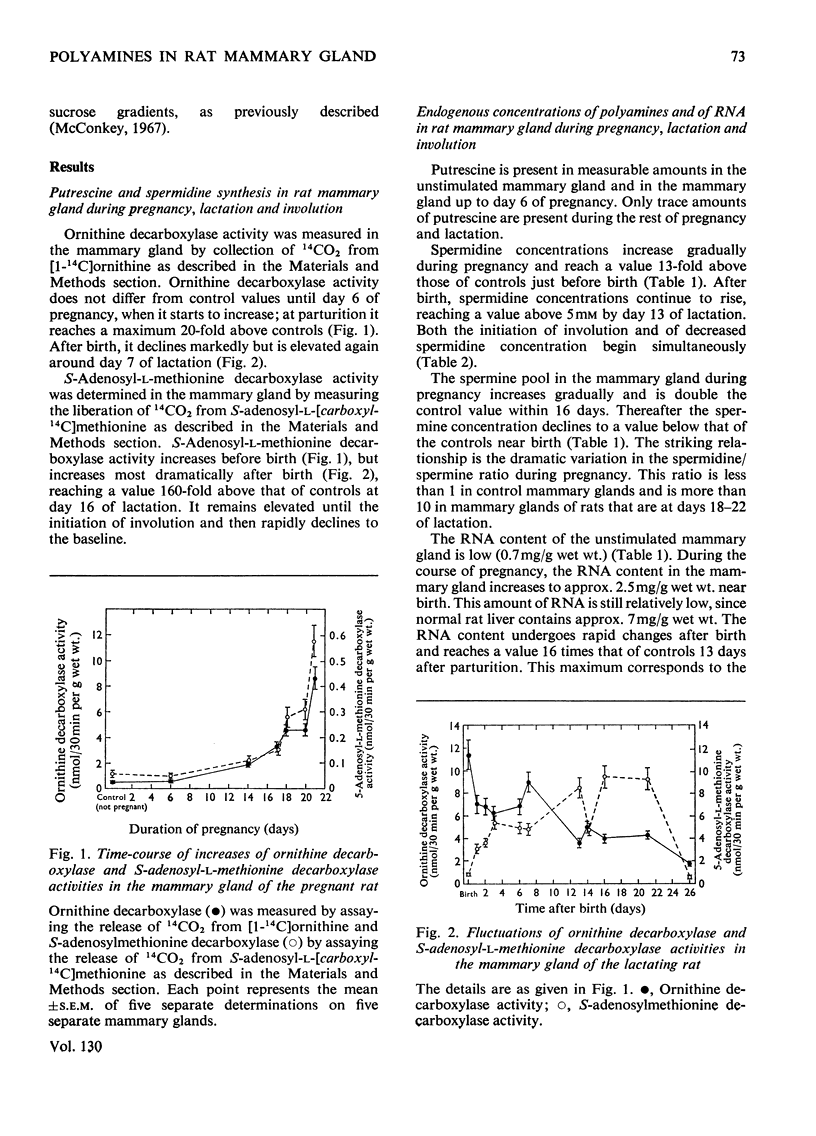
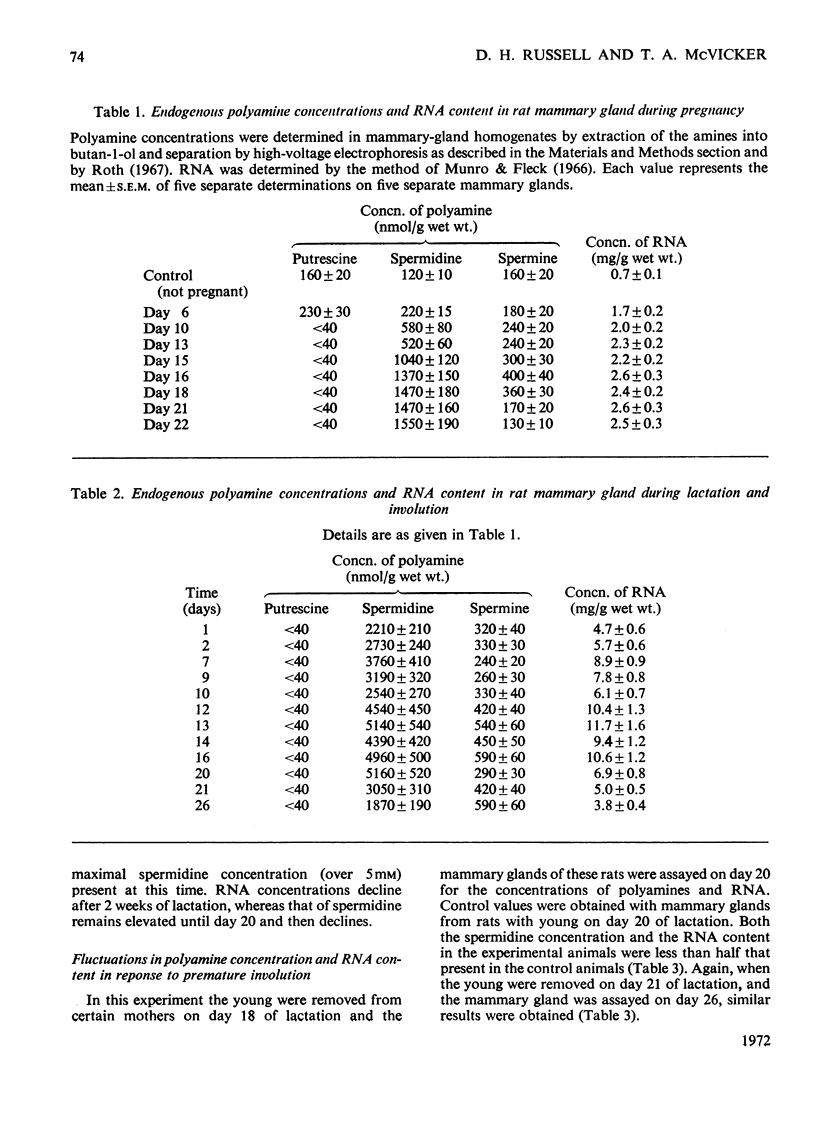
Polyamines and RNA accumulate in the rat mammary gland during pregnancy, but the major increases occur after parturition. Therefore the major increases occur after the gland has obtained its maximal complement of epithelial cells. During lactation, the spermidine concentration rises above 5mm and RNA content in the lactating mammary gland reaches a value 16 times that of the unstimulated mammary gland. The ratio of spermidine/spermine, an increase of which initially signals an elevation in biosynthetic activity, is near 1 in the normal mammary gland and is greater than 10 in the lactating mammary gland. Putrescine concentration is very low during the entire course of mammary-gland development, with the exception of early pregnancy. The low putrescine concentration probably reflects the very rapid conversion of putrescine into spermidine. Both ornithine decarboxylase, the enzyme that synthesizes putrescine, and putrescine-stimulated S-adenosyl-l-methionine decarboxylase, the enzyme that synthesizes spermidine, increase in activity during middle and late pregnancy; during lactation, both enzyme activities are elevated until the 21st day of lactation, and then decline. These declines are concomitant with involution. Also, it was found that the amount of ribonuclease activity in the mammary gland was very high during lactation, almost double that in the gland during pregnancy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., SMITH K. C., ALLEN F. W. The preparation and characterization of ribonucleic acids from yeast. J Biol Chem. 1955 Sep;216(1):185–193. [PubMed] [Google Scholar]
- Gfeller E., Russell D. H. Autoradiographic distributions of 3 H-putrescine and 3 H-uridine in a Xenopus liver cell line. Z Zellforsch Mikrosk Anat. 1971;120(3):321–331. doi: 10.1007/BF00324895. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Neish W. J., Key L. Polyamines and glutathione in tissues of lactating rats (Rattus rattus) and in the Rd-3 adcites tumour. Comp Biochem Physiol. 1968 Dec;27(3):709–714. doi: 10.1016/0010-406x(68)90611-7. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Pelc S. R. Turnover of DNA and function. Nature. 1968 Jul 13;219(5150):162–163. doi: 10.1038/219162a0. [DOI] [PubMed] [Google Scholar]
- Raina A., Cohen S. S. Polyamines and RNA synthesis in a polyauxotrophic strain of E. coli. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1587–1593. doi: 10.1073/pnas.55.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Levy C. C., Taylor R. L. Increased DNA associated with rat liver nucleoli when isolated with spermidine. Biochem Biophys Res Commun. 1972 Apr 14;47(1):212–217. doi: 10.1016/s0006-291x(72)80030-5. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Lombardini J. P. Polyamines. (1) Enhanced s-adenosyl-L-methionine decarboxylase in rapid growth systems, and (2) the relationships between polyamine concentration and RNA accumulation. Biochim Biophys Acta. 1971 Jun 30;240(2):273–286. doi: 10.1016/0005-2787(71)90666-6. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Putrescine and spermidine biosynthesis in the development of normal and anucleolate mutants of Xenopus laevis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):523–527. doi: 10.1073/pnas.68.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: effect of hypophysectomy and growth hormone on ornithine decarboxylase. Endocrinology. 1969 Feb;84(2):223–228. doi: 10.1210/endo-84-2-223. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. A., Schmidt G. H. Nucleic acid content of rat mammary gland nucleic during pregnancy, lactation, and involution. Proc Soc Exp Biol Med. 1969 Dec;132(3):978–983. doi: 10.3181/00379727-132-34349. [DOI] [PubMed] [Google Scholar]
- Sod-Moriah U. A., Schmidt G. H. Deoxyribonucleic acid content and proliferative activity of rabbit mammary gland epithelial cells. Exp Cell Res. 1968 Mar;49(3):584–597. doi: 10.1016/0014-4827(68)90206-1. [DOI] [PubMed] [Google Scholar]


