Abstract
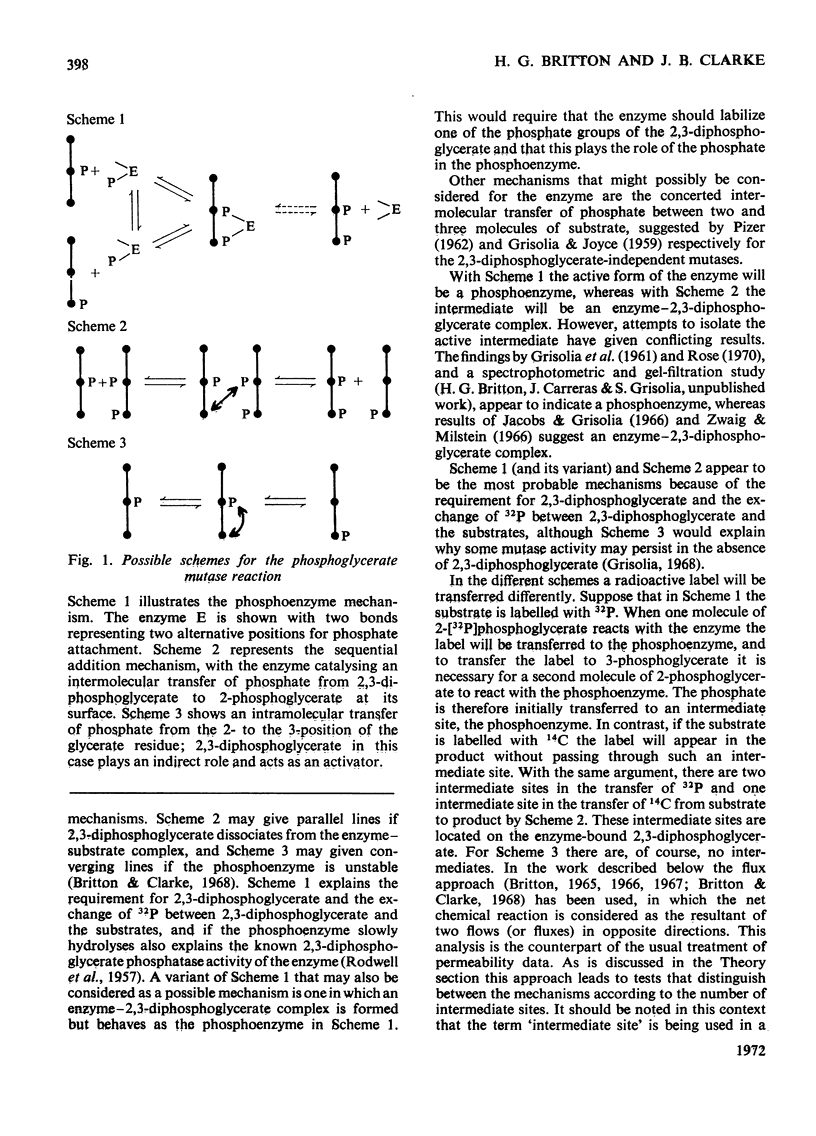
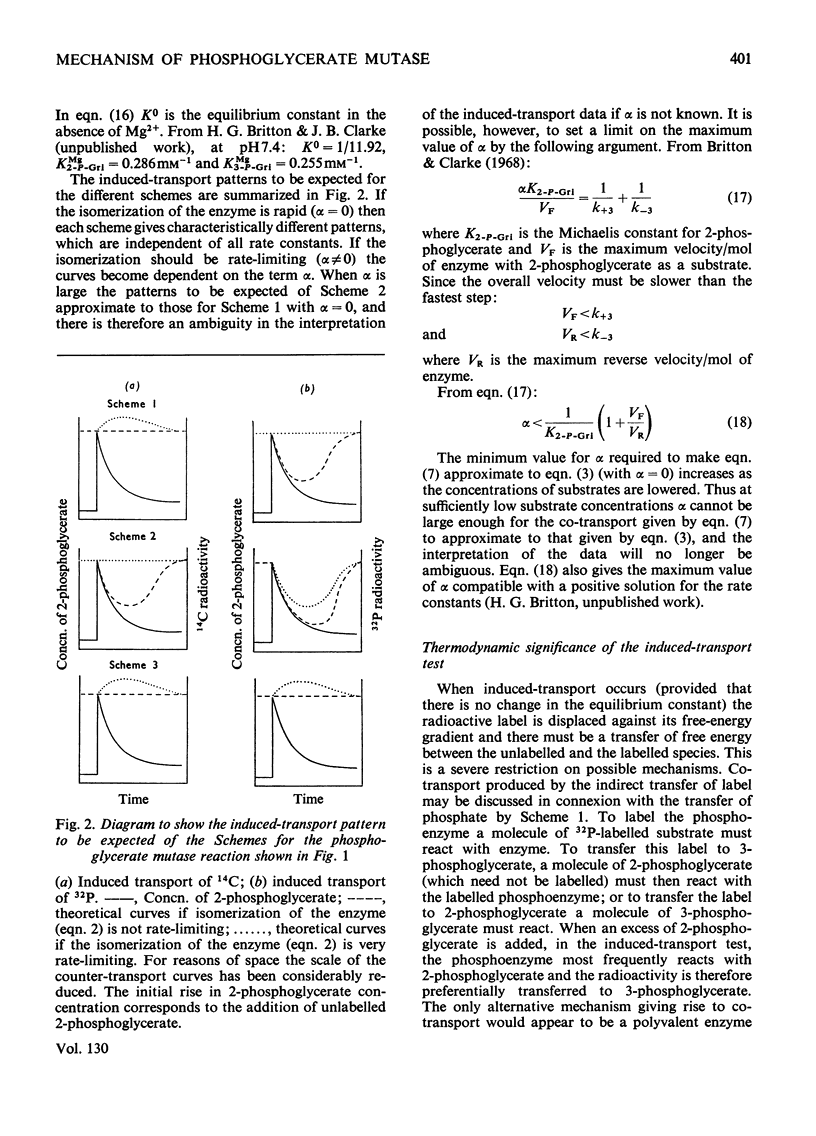
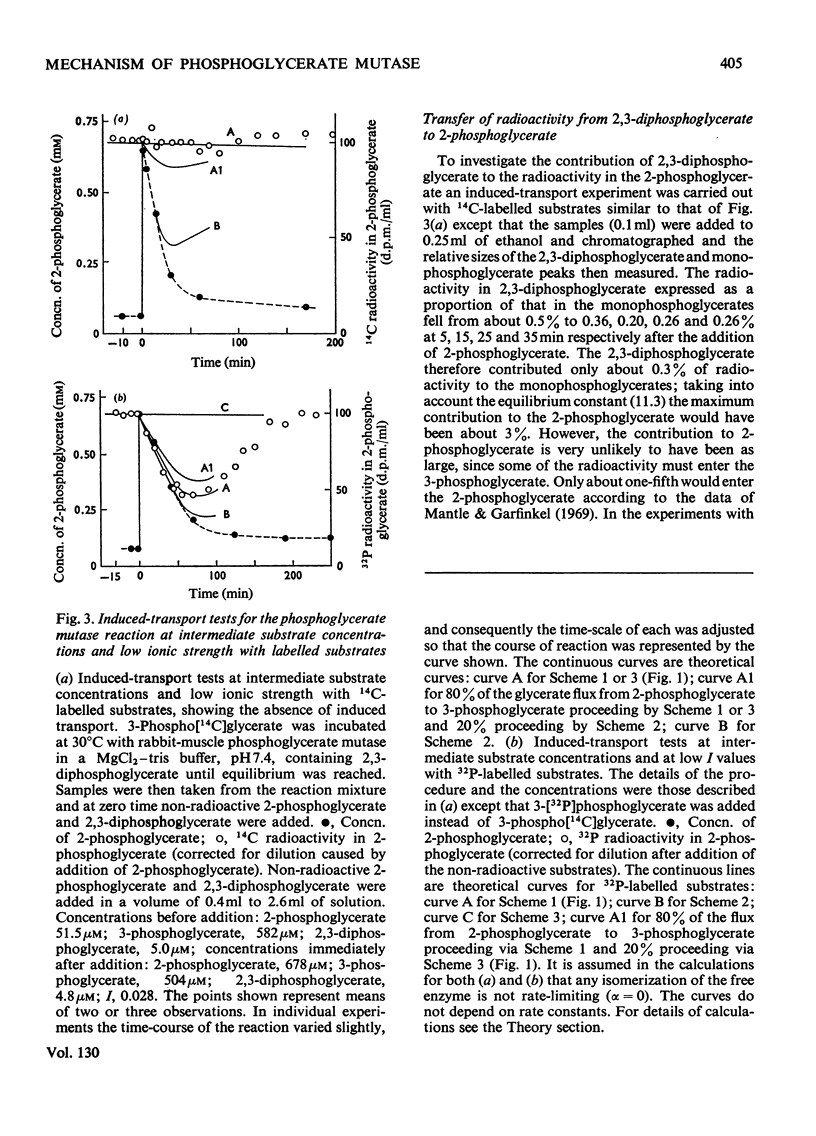
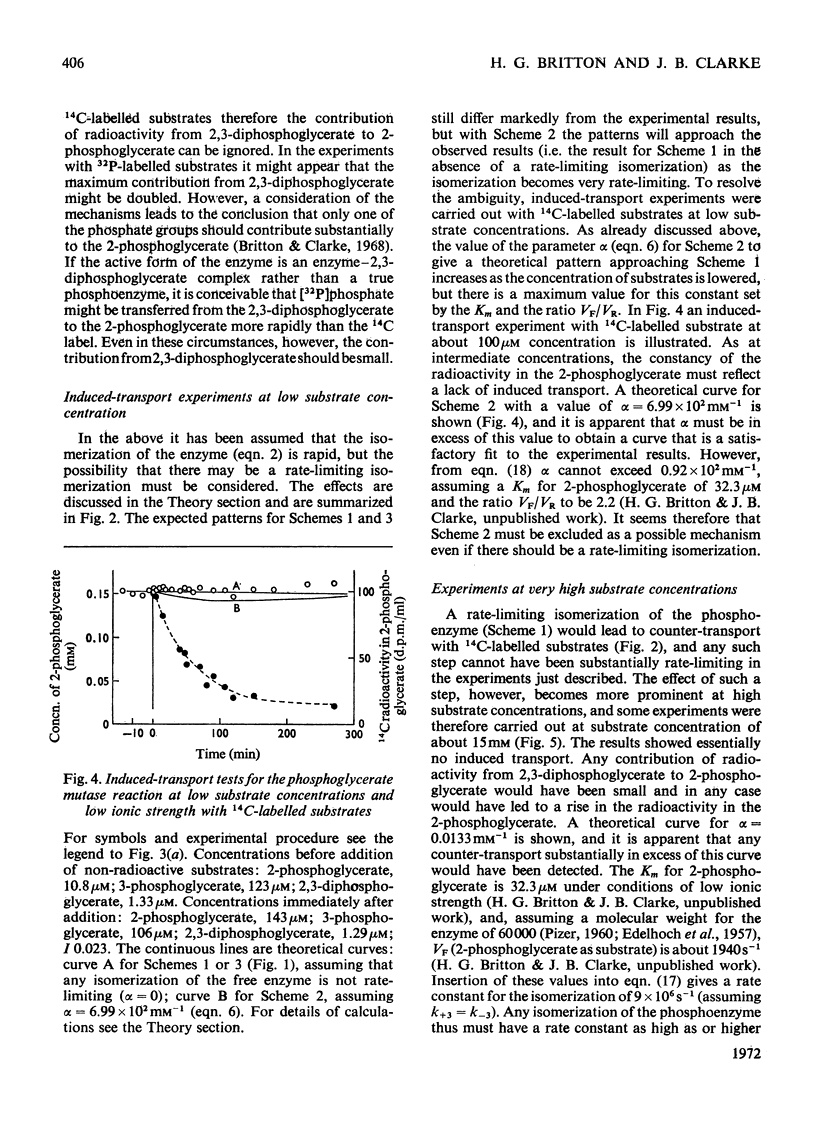
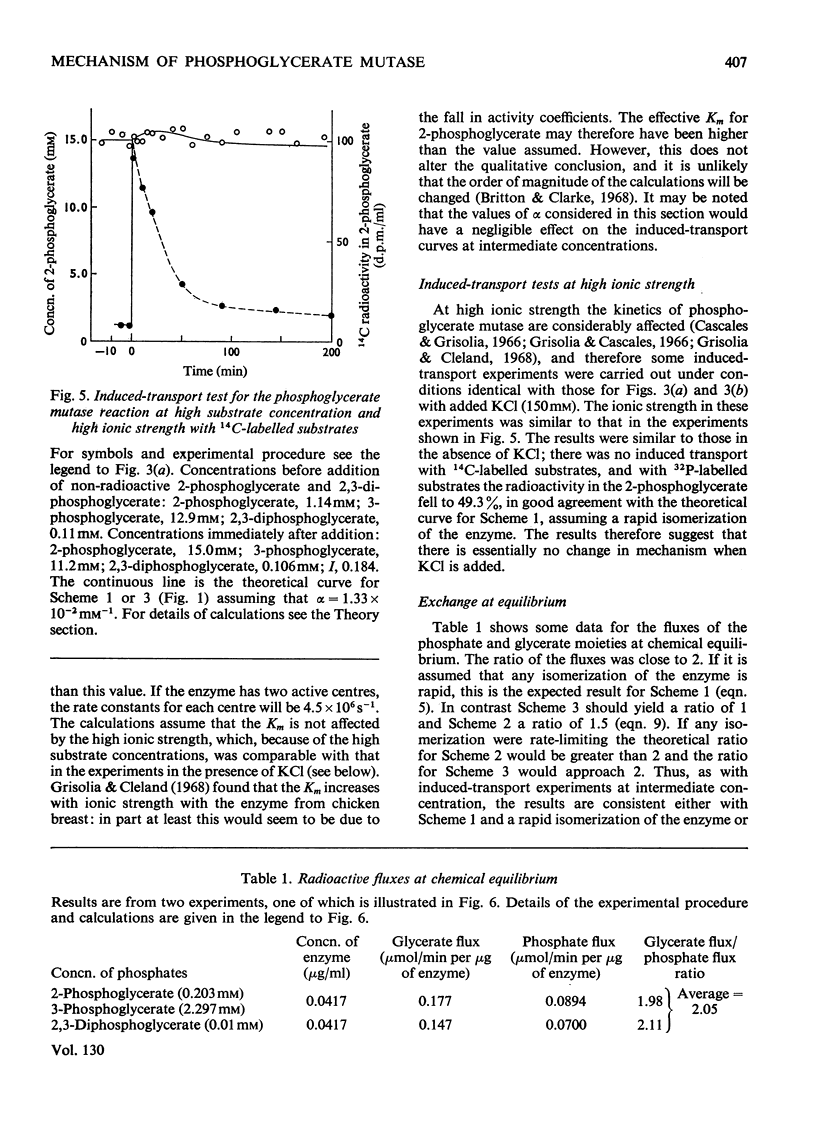
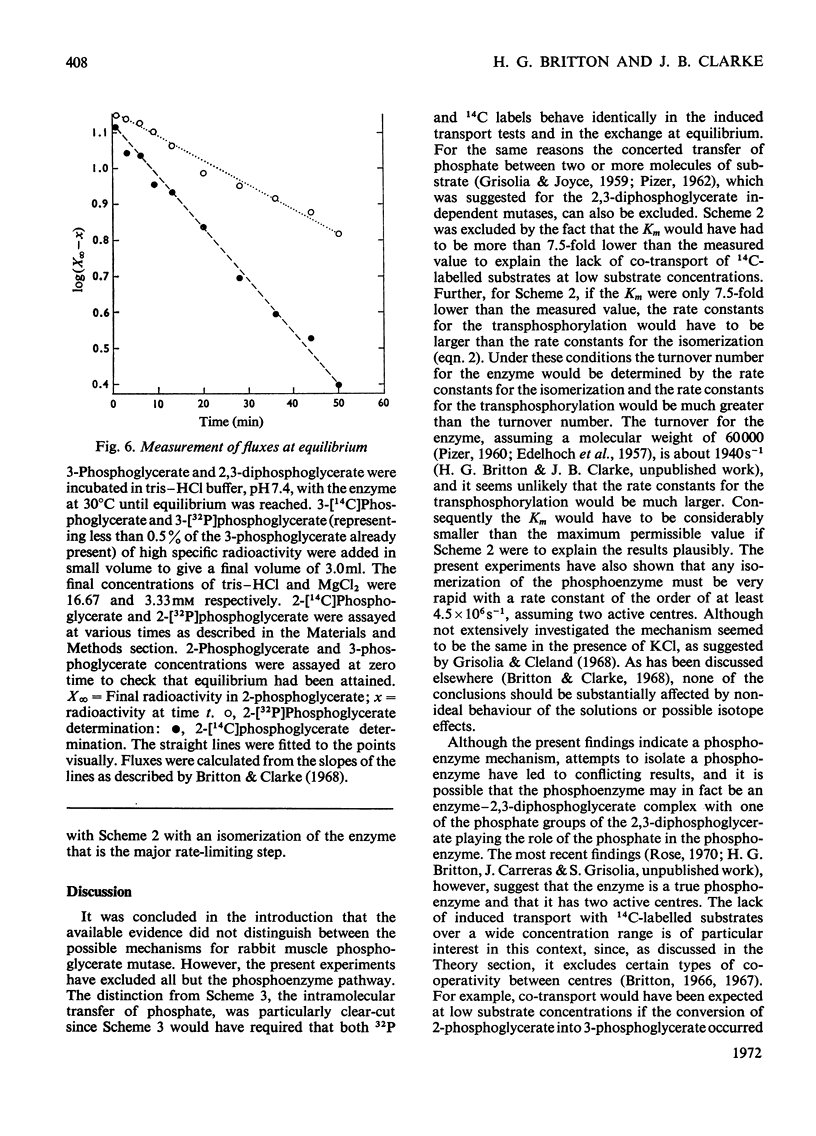
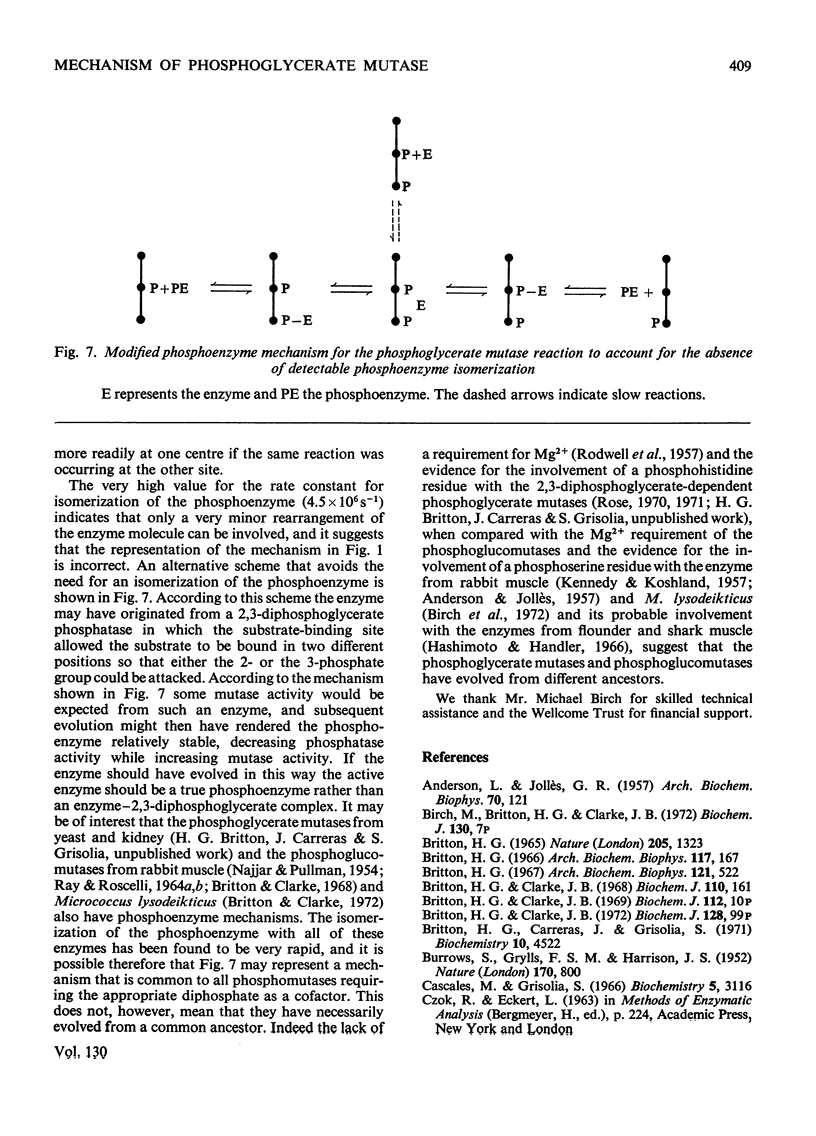
1. The properties and kinetics of the 2,3-diphosphoglycerate-dependent phosphoglycerate mutases are discussed. There are at least three possible mechanisms for the reaction: (i) a phosphoenzyme (Ping Pong) mechanism; (ii) an intermolecular transfer of phosphate from 2,3-diphosphoglycerate to the substrates (sequential mechanism); (iii) an intramolecular transfer of phosphate. It is concluded that these mechanisms cannot be distinguished by conventional kinetic measurements. 2. The fluxes for the different mechanisms are calculated and it is shown that it should be possible to distinguish between the mechanisms by appropriate induced-transport tests and by comparing the fluxes of 32P- and 14C-labelled substrates at chemical equilibrium. 3. With 14C-labelled substrates no induced transport was found over a wide concentration range, and with 32P-labelled substrates co-transport occurred that was independent of concentration over a twofold range. 14C-labelled substrates exchange at twice the rate of 32P-labelled substrates at chemical equilibrium. The results were completely in accord with a phosphoenzyme mechanism and indicated a rate constant for the isomerization of the phosphoenzyme of not less than 4×106s−1. The intramolecular transfer of phosphate (and intermolecular transfer between two or more molecules of substrate) were completely excluded. The intermolecular transfer of phosphate from 2,3-diphosphoglycerate would have been compatible with the results only if the Km for 2-phosphoglycerate had been over 7.5-fold smaller than the observed value and if an isomerization of the enzyme-2,3-diphosphoglycerate complex had been the major rate-limiting step in the reaction. 4. The very rapid isomerization of the phosphoenzyme that the experiments demonstrate suggests a mechanism that does not involve a formal isomerization. According to this new scheme the enzyme is closely related mechanistically and perhaps evolutionarily to a 2,3-diphosphoglycerate diphosphatase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON L., JOLLES G. R. A study of the linkage of phosphorus to protein in phosphoglucomutase. Arch Biochem Biophys. 1957 Jul;70(1):121–128. doi: 10.1016/0003-9861(57)90085-1. [DOI] [PubMed] [Google Scholar]
- BRITTON H. G. THE USSING RELATIONSHIP AND CHEMICAL REACTIONS: POSSIBLE APPLICATION TO ENZYMATIC INVESTIGATIONS. Nature. 1965 Mar 27;205:1323–1324. doi: 10.1038/2051323b0. [DOI] [PubMed] [Google Scholar]
- Britton H. G., Carreras J., Grisolia S. Mechanism of action of 2,3-diphosphoglycerate-independent phosphoglycerate mutase. Biochemistry. 1971 Nov 23;10(24):4522–4533. doi: 10.1021/bi00800a028. [DOI] [PubMed] [Google Scholar]
- Britton H. G., Clarke J. B. The mechanism of the phosphoglucomutase reaction. Studies on rabbit muscle phosphoglucomutase with flux techniques. Biochem J. 1968 Nov;110(2):161–180. doi: 10.1042/bj1100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton H. G. Communications. Arch Biochem Biophys. 1967 Aug;121(2):522–522. doi: 10.1016/0003-9861(67)90108-7. [DOI] [PubMed] [Google Scholar]
- Britton H. G. The concept and use of flux measurements in enzyme studies. A theoretical analysis. Arch Biochem Biophys. 1966 Oct;117(1):167–183. doi: 10.1016/0003-9861(66)90140-8. [DOI] [PubMed] [Google Scholar]
- Cascales M., Grisolia S. Influence of ionic strength on apparent reaction mechanism of phosphoglycerate mutase. Biochemistry. 1966 Oct;5(10):3116–3122. doi: 10.1021/bi00874a006. [DOI] [PubMed] [Google Scholar]
- Dunstan P. M., Anthony C., Drabble W. T. Microbial metabolism of C 1 and C 2 compounds. The involvement of glycollate in the metabolism of ethanol and of acetate by Pseudomonas AM1. Biochem J. 1972 Jun;128(1):99–106. doi: 10.1042/bj1280099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELHOCH H., RODWELL V. W., GRISOLIA S. The molecular properties of yeast and muscle phosphoglyceric acid mutase. J Biol Chem. 1957 Oct;228(2):891–903. [PubMed] [Google Scholar]
- EGGLESTON L. V., HEMS R. Separation of adenosine phosphates by paper chromotography and the equilibrium constant of the myokinase system. Biochem J. 1952 Sep;52(1):156–160. doi: 10.1042/bj0520156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRISOLIA S., JOYCE B. K. Distribution of two types of phosphoglyceric acid mutase, diphosphoglycerate mutase, and D-2,3-diphosphoglyceric acid. J Biol Chem. 1959 Jun;234(6):1335–1337. [PubMed] [Google Scholar]
- GRISOLIA S., JOYCE B. K., FERNANDEZ M. Studies on the mechanism of action of phosphoglyceromutases. Biochim Biophys Acta. 1961 Jun 10;50:81–89. doi: 10.1016/0006-3002(61)91063-0. [DOI] [PubMed] [Google Scholar]
- Grisolia S., Cascales M. Apparent change in reaction mechanism of phosphoglycerate mutase induced by salt. Biochem Biophys Res Commun. 1966 Jan 24;22(2):200–205. doi: 10.1016/0006-291x(66)90432-3. [DOI] [PubMed] [Google Scholar]
- Grisolia S., Cleland W. W. Influence of salt, substrate, and cofactor concentrations on the kinetic and mechanistic behavior of phosphoglycerate mutase. Biochemistry. 1968 Mar;7(3):1115–1121. doi: 10.1021/bi00843a032. [DOI] [PubMed] [Google Scholar]
- HARRISON W. H., BOYER P. D., FALCONE A. B. The mechanism of enzymic phosphate transfer reactions. J Biol Chem. 1955 Jul;215(1):303–317. [PubMed] [Google Scholar]
- Hashimoto T., Handler P. Phosphoglucomutase. 3. Purification and properties of phosphoglucomutases from flounder and shark muscle. J Biol Chem. 1966 Sep 10;241(17):3940–3948. [PubMed] [Google Scholar]
- Jacobs R. J., Grisolia S. Phosphoryl intermediates formed with phosphoglycerate mutase. Role and labilization of 2,3-diphosphoglycerate. J Biol Chem. 1966 Dec 25;241(24):5926–5935. [PubMed] [Google Scholar]
- KENNEDY E. P., KOSHLAND D. E., Jr Properties of the phosphorylated active site of phosphoglucomutase. J Biol Chem. 1957 Sep;228(1):419–431. [PubMed] [Google Scholar]
- Mantle J., Garfinkel D. Phosphoglyceric acid mutase. A computer simulation study. J Biol Chem. 1969 Jul 25;244(14):3884–3889. [PubMed] [Google Scholar]
- NAJJAR V. A., PULLMAN M. E. The occurrence of a group transfer involving enzyme (phosphoglucomutase) and substrate. Science. 1954 May 7;119(3097):631–634. doi: 10.1126/science.119.3097.631. [DOI] [PubMed] [Google Scholar]
- PIZER L. I., BALLOU C. E. Specificity of phosphoglyceric acid mutase. J Biol Chem. 1959 May;234(5):1138–1142. [PubMed] [Google Scholar]
- PIZER L. I. Properties of the phosphoprotein-phosphoglyceric mutase. J Biol Chem. 1960 Apr;235:895–901. [PubMed] [Google Scholar]
- RAY W. J., Jr, ROSCELLI G. A. THE PHOSPHOGLUCOMUTASE PATHWAY. AN INVESTIGATION OF PHOSPHO-ENZYME ISOMERIZATION. J Biol Chem. 1964 Nov;239:3935–3941. [PubMed] [Google Scholar]
- RODWELL V. W., TOWNE J. C., GRISOLIA S. The kinetic properties of yeast and muscle phosphoglyceric acid mutase. J Biol Chem. 1957 Oct;228(2):875–890. [PubMed] [Google Scholar]
- Rose Z. B. Evidence for a phosphohistidine protein intermediate in the phosphoglycerate mutase reaction. Arch Biochem Biophys. 1970 Oct;140(2):508–513. doi: 10.1016/0003-9861(70)90095-0. [DOI] [PubMed] [Google Scholar]
- Rose Z. B. The phosphorylation of yeast phosphoglycerate mutase. Arch Biochem Biophys. 1971 Sep;146(1):359–360. doi: 10.1016/s0003-9861(71)80076-0. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- ZWAIG N., MILSTEIN C. ON THE NATURE OF THE PHOSPHOENZYME INTERMEDIATE IN THE PHOSPHOGLYCEROMUTASE REACTION. Biochim Biophys Acta. 1963 Aug 6;73:676–679. doi: 10.1016/0006-3002(63)90347-0. [DOI] [PubMed] [Google Scholar]
- Zwaig N., Milstein C. The phosphorylated intermediate in the phosphoglyceromutase reaction. Biochem J. 1966 Feb;98(2):360–368. doi: 10.1042/bj0980360. [DOI] [PMC free article] [PubMed] [Google Scholar]


