Abstract
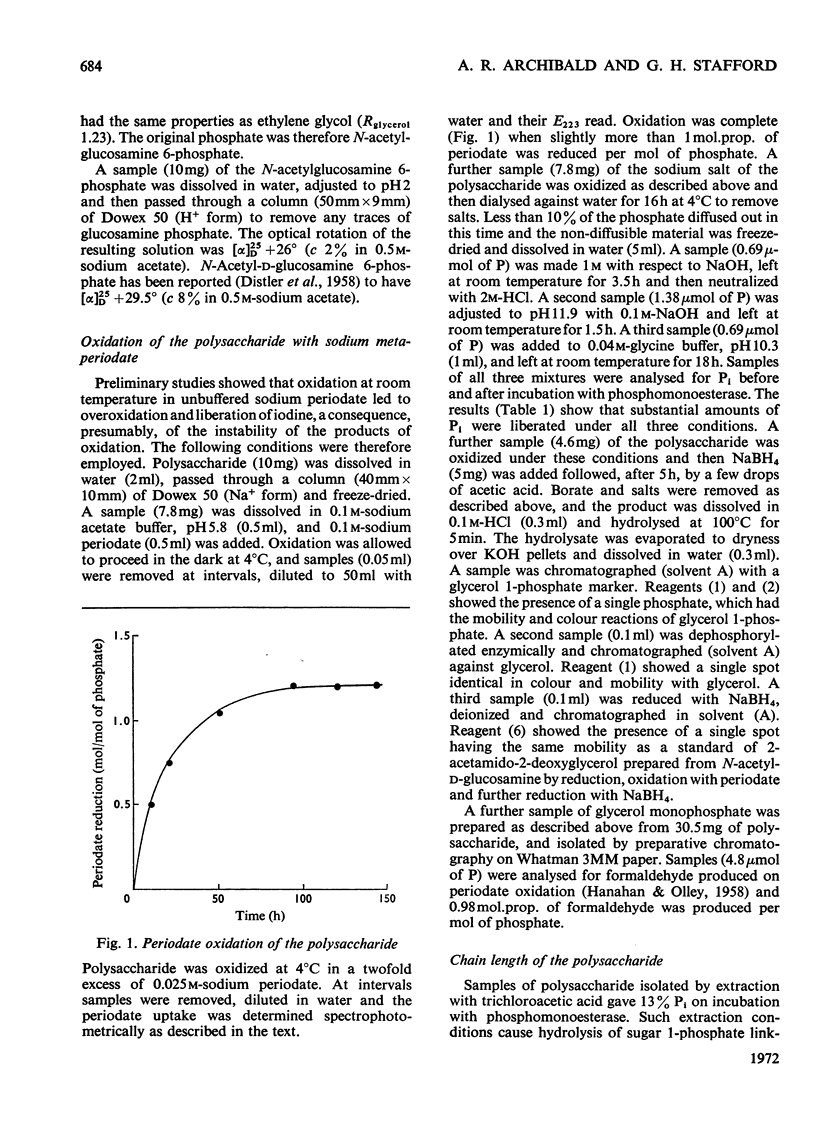
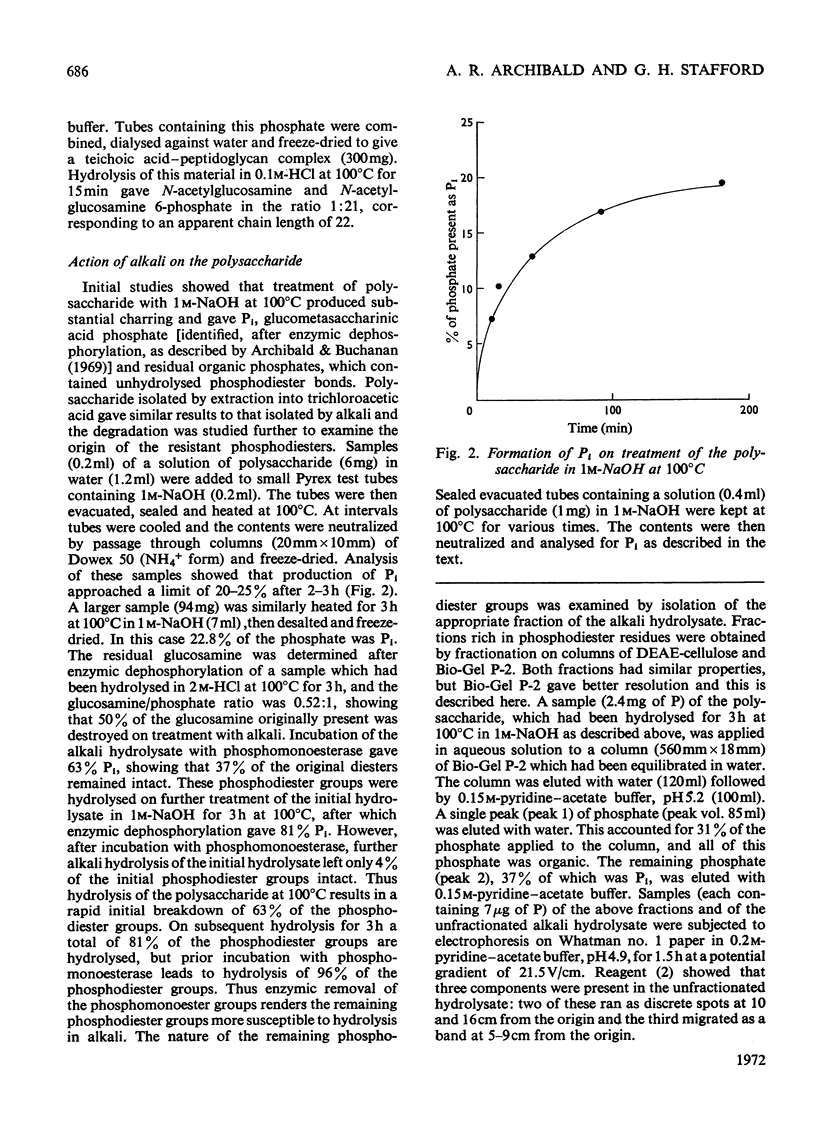
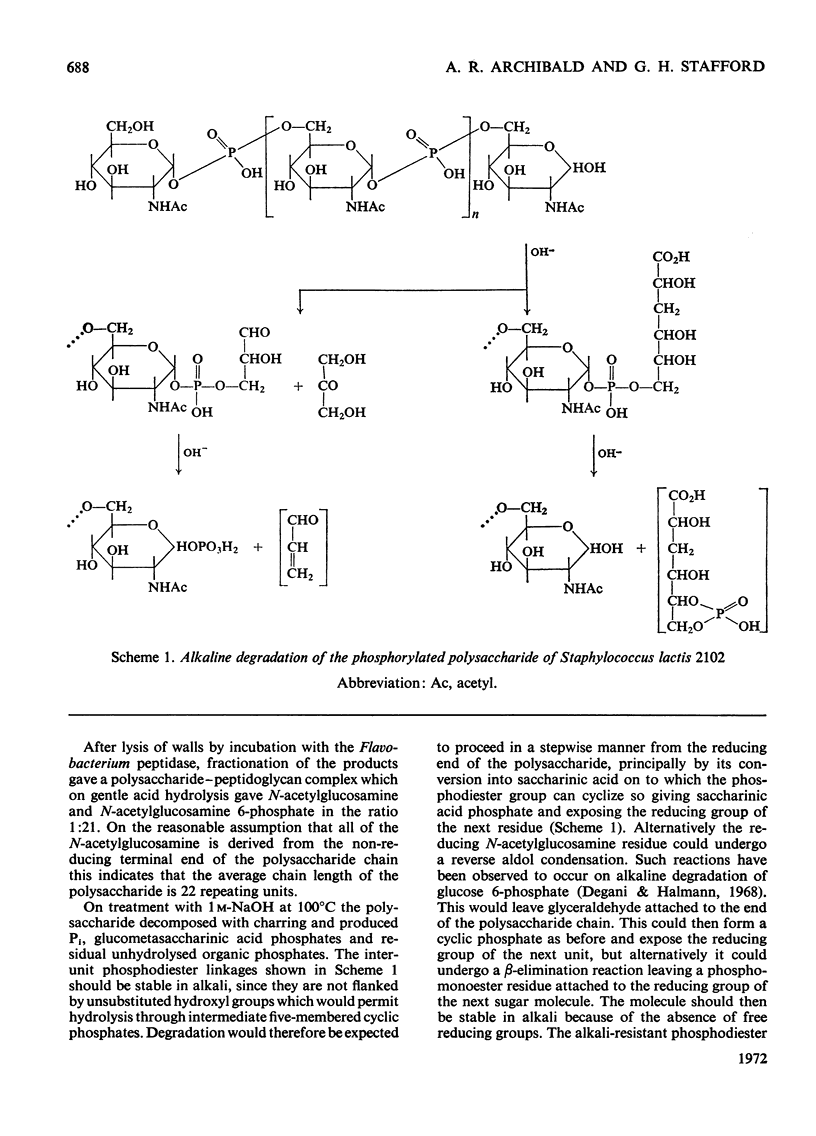
1. Walls of Staphylococcus lactis 2102 contain about 40% of a phosphorylated polysaccharide, which was isolated by extraction with cold trichloroacetic acid, with dilute NaOH, and also by digestion with a Flavobacterium peptidase. 2. The purified polymer contained equimolar proportions of N-acetylglucosamine and phosphate as its sole constituents and was readily hydrolysed under gentle acidic conditions to N-acetylglucosamine 6-phosphate. 3. Studies on the intact polymer showed that it is linear and that adjacent acetamido sugar units are joined by phosphodiester bonds between their 1- and 6-positions, the glycosidic linkages having the α-configuration. This polymer is thus the simplest of the known microbial wall polymers possessing sugar 1-phosphate linkages. 4. Alkali degradation of the extracted polymer proceeds predominantly in a stepwise manner from the reducing end, but evidence was obtained for the direct hydrolysis of some of the inter-unit phosphodiester groups.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Baddiley J., Button D., Heptinstall S., Stafford G. H. Ocuurrence of polymers containing N-acetylglucosamine-1-phosphate in bacterial walls. Nature. 1968 Aug 24;219(5156):855–856. doi: 10.1038/219855a0. [DOI] [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Button D. The glycerol teichoic acid of walls of Staphylococcus lactis I3. Biochem J. 1968 Dec;110(3):543–557. doi: 10.1042/bj1100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Heckels J. E., Heptinstall S. Further studies on the glycerol teichoic acid of walls of Staphylococcus lactis I3. Location of the phosphodiester groups and their susceptibility to hydrolysis with alkali. Biochem J. 1971 Nov;125(1):353–359. doi: 10.1042/bj1250353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Coapes H. E., Stafford G. H. The action of dilute alkali on bacterial cell walls. Biochem J. 1969 Aug;113(5):899–900. doi: 10.1042/bj1130899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Heptinstall S. The teichoic acids of Micrococcus sp. 24. Biochem J. 1971 Nov;125(1):361–363. doi: 10.1042/bj1250361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREITENBACH J. W., DERKOSCH J., WESSELY F. Energetics of peptide formation. Nature. 1952 May 31;169(4309):922–922. doi: 10.1038/169922a0. [DOI] [PubMed] [Google Scholar]
- Brooks D., Baddiley J. A lipid intermediate in the synthesis of a poly-(N-acetylglucosamine 1-phosphate) from the wall of Staphylococcus lactis N.C.T.C. 2102. Biochem J. 1969 Nov;115(2):307–314. doi: 10.1042/bj1150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISTLER J. J., MERRICK J. M., ROSEMAN S. Glucosamine metabolism. III. Preparation and N-acetylation of crystalline D-glucosamine- and D-galactosamine-6-phosphoric acids. J Biol Chem. 1958 Jan;230(1):497–509. [PubMed] [Google Scholar]
- Davison A. L. The characterization of a Micrococcus (Staphylococcus lactis) sp. I3 with an atypical teichoic acid in its wall. Biochem J. 1968 Dec;110(3):557–558. doi: 10.1042/bj1100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAHAN D. J., OLLEY J. N. Chemical nature of monophosphoinositides. J Biol Chem. 1958 Apr;231(2):813–828. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J. The action of dilute alkali on some bacterial cell walls. Biochem Biophys Res Commun. 1968 Oct 10;33(1):22–28. doi: 10.1016/0006-291x(68)90248-9. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Linkage between the teichuronic acid and mucopeptide components. Biochem J. 1970 Apr;117(3):431–439. doi: 10.1042/bj1170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey H., Brooks D., Baddiley J. Direction of chain extension during the biosynthesis of teichoic acids in bacterial cell walls. Nature. 1969 Feb 15;221(5181):665–666. doi: 10.1038/221665a0. [DOI] [PubMed] [Google Scholar]
- Kato K., Strominger J. L. Structure of the cell wall of Staphylococcus aureaus. IX. Mechanism of hydrolysis by the L11 enzyme. Biochemistry. 1968 Aug;7(8):2745–2761. [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Partridge M. D., Davison A. L., Baddiley J. A polymer of glucose and N-acetylgalactosamine 1-phosphate in the wall of Micrococcus sp. A1. Biochem J. 1971 Feb;121(4):695–700. doi: 10.1042/bj1210695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Slodki M. E. The structure of extracellular phosphorylated galactans from Sporobolomyces yeasts. J Biol Chem. 1966 Jun 10;241(11):2700–2706. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WICKERHAM L. J., BURTON K. A. Phylogeny and biochemistry of the genus Hansenula. Bacteriol Rev. 1962 Dec;26:382–397. doi: 10.1128/br.26.4.382-397.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Woods D. D. The synthesis of p-aminobenzoic acid and folic acid by staphylococci sensitive and resistant to sulphonamides. J Gen Microbiol. 1965 Aug;40(2):243–253. doi: 10.1099/00221287-40-2-243. [DOI] [PubMed] [Google Scholar]


