Abstract
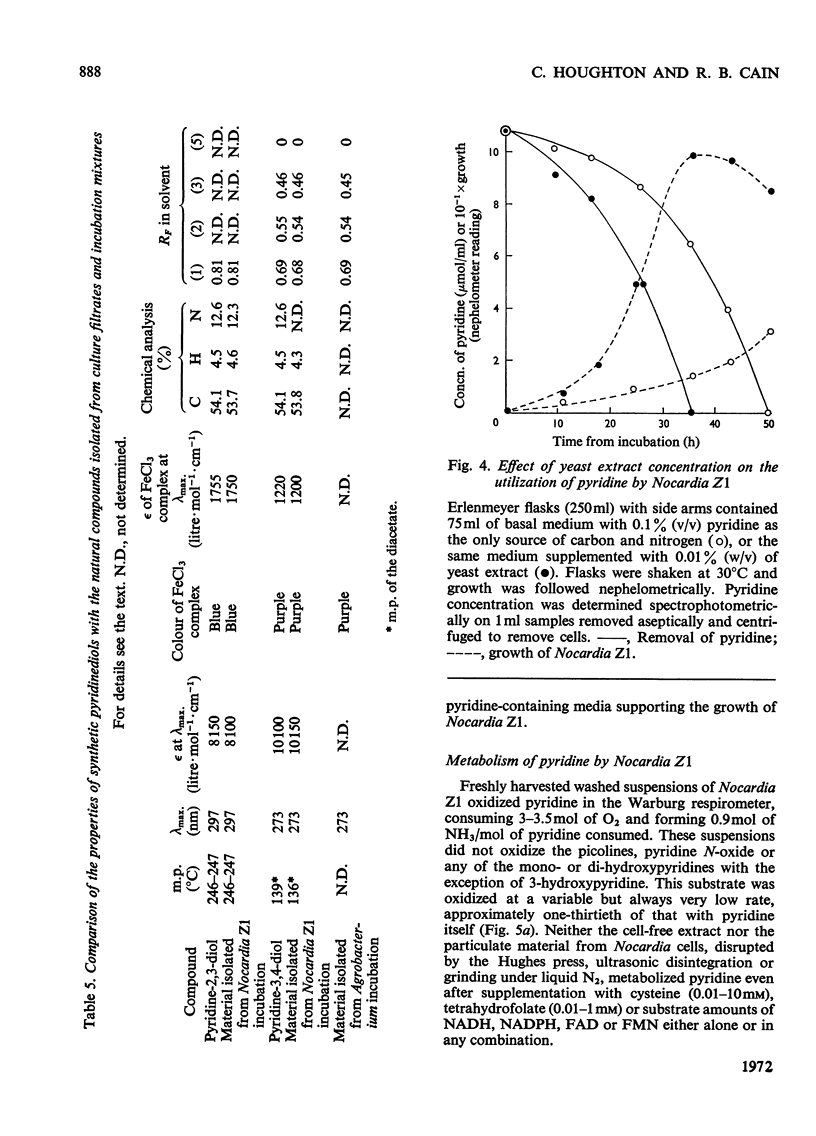
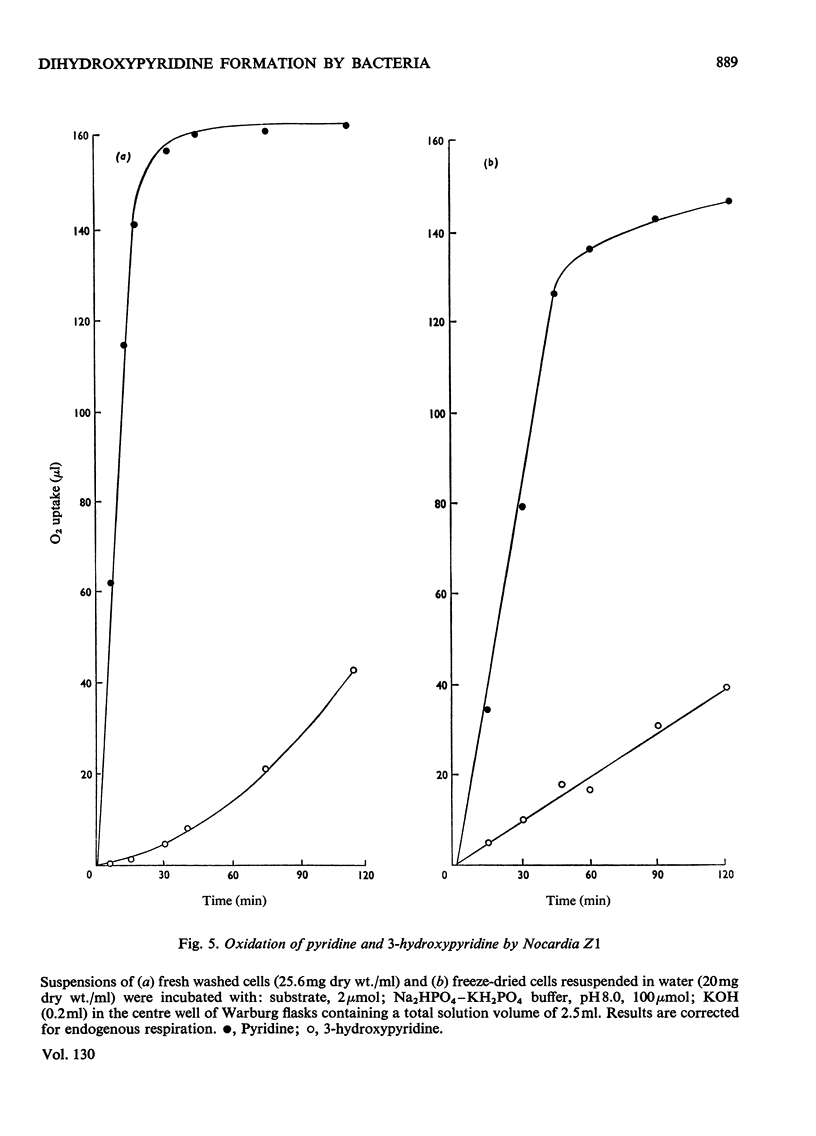
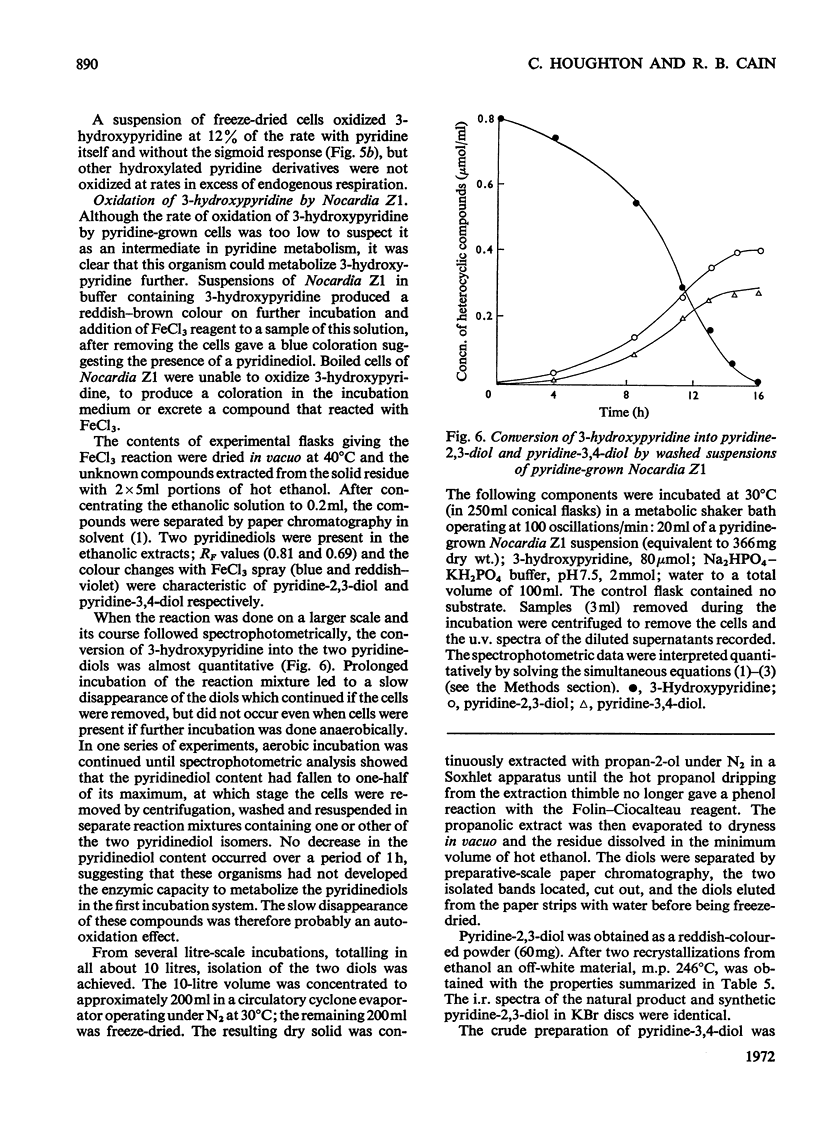
1. Several species of micro-organisms that were capable of utilizing pyridine compounds as carbon and energy source were isolated from soil and sewage. Compounds degraded included pyridine and the three isomeric hydroxypyridines. 2. Suitable modifications of the cultural conditions led to the accumulation of pyridinediols (dihydroxypyridines), which were isolated and characterized. 3. Three species of Achromobacter produced pyridine-2,5-diol from 2- or 3-hydroxypyridine whereas an uncommon Agrobacterium sp. (N.C.I.B. 10413) produced pyridine-3,4-diol from 4-hydroxypyridine. 4. On the basis of chemical isolation, induction of the necessary enzymes in washed suspensions and the substrate specificity exhibited by the isolated bacteria, the initial transformations proposed are: 2-hydroxypyridine → pyridine-2,5-diol; 3-hydroxypyridine → pyridine-2,5-diol and 4-hydroxypyridine → pyridine-3,4-diol. 5. A selected pyridine-utilizer, Nocardia Z1, did not produce any detectable hydroxy derivative from pyridine, but carried out a slow oxidation of 3-hydroxypyridine to pyridine-2,3-diol and pyridine-3,4-diol. These diols were not further metabolized. 6. Addition of the isomeric hydroxypyridines to a model hydroxylating system resulted in the formation of those diols predicted by theory.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRESLOW R., LUKENS L. N. On the mechanism of action of an ascorbic acid-dependent nonenzymatic hydroxylating system. J Biol Chem. 1960 Feb;235:292–296. [PubMed] [Google Scholar]
- Bade M., Gould B. S. Proline hydroxylation by oxygen. Biochim Biophys Acta. 1968 Mar 11;156(2):425–428. doi: 10.1016/0304-4165(68)90279-1. [DOI] [PubMed] [Google Scholar]
- Cain R. B., Bilton R. F., Darrah J. A. The metabolism of aromatic acids by micro-organisms. Metabolic pathways in the fungi. Biochem J. 1968 Aug;108(5):797–828. doi: 10.1042/bj1080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderbank A. The bipyridylium herbicides. Adv Pest Control Res. 1968;8:127–235. [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- HUNT A. L., HUGHES D. E., LOWENSTEIN J. M. The hydroxylation of nicotinic acid by Pseudomonas fluorescens. Biochem J. 1958 Jun;69(2):170–173. doi: 10.1042/bj0690170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O. Enzymic hydroxylation. Annu Rev Biochem. 1969;38:21–44. doi: 10.1146/annurev.bi.38.070169.000321. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I., Dalton B. P. The hydroxylation of nicotine: the origin of the hydroxyl oxygen. Biochem Biophys Res Commun. 1965 Dec 21;21(6):644–648. doi: 10.1016/0006-291x(65)90535-8. [DOI] [PubMed] [Google Scholar]
- Mulder G. J. The heterogeneity of uridine diphosphate glucuronyltransferase from rat liver. Biochem J. 1971 Nov;125(1):9–15. doi: 10.1042/bj1250009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Biochemical changes occurring during the germination of bacterial spores. Biochem J. 1953 May;54(2):205–209. doi: 10.1042/bj0540205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UDENFRIEND S., CLARK C. T., AXELROD J., BRODIE B. B. Ascorbic acid in aromatic hydroxylation. I. A model system for aromatic hydroxylation. J Biol Chem. 1954 Jun;208(2):731–739. [PubMed] [Google Scholar]
- Wright K. A., Cain R. B. Microbial metabolism of pyridinium compounds. Metabolism of 4-carboxy-1-methylpyridinium chloride, a photolytic product of paraquat. Biochem J. 1972 Jul;128(3):543–559. doi: 10.1042/bj1280543. [DOI] [PMC free article] [PubMed] [Google Scholar]


