Abstract
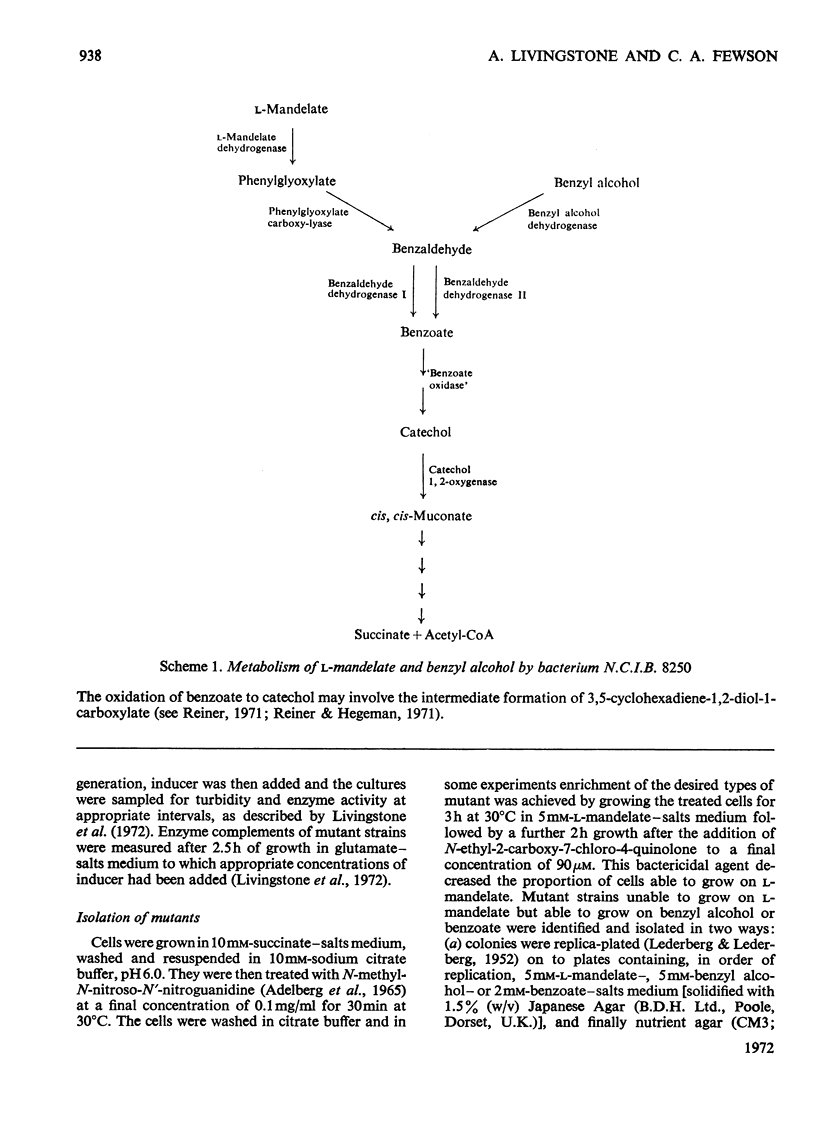
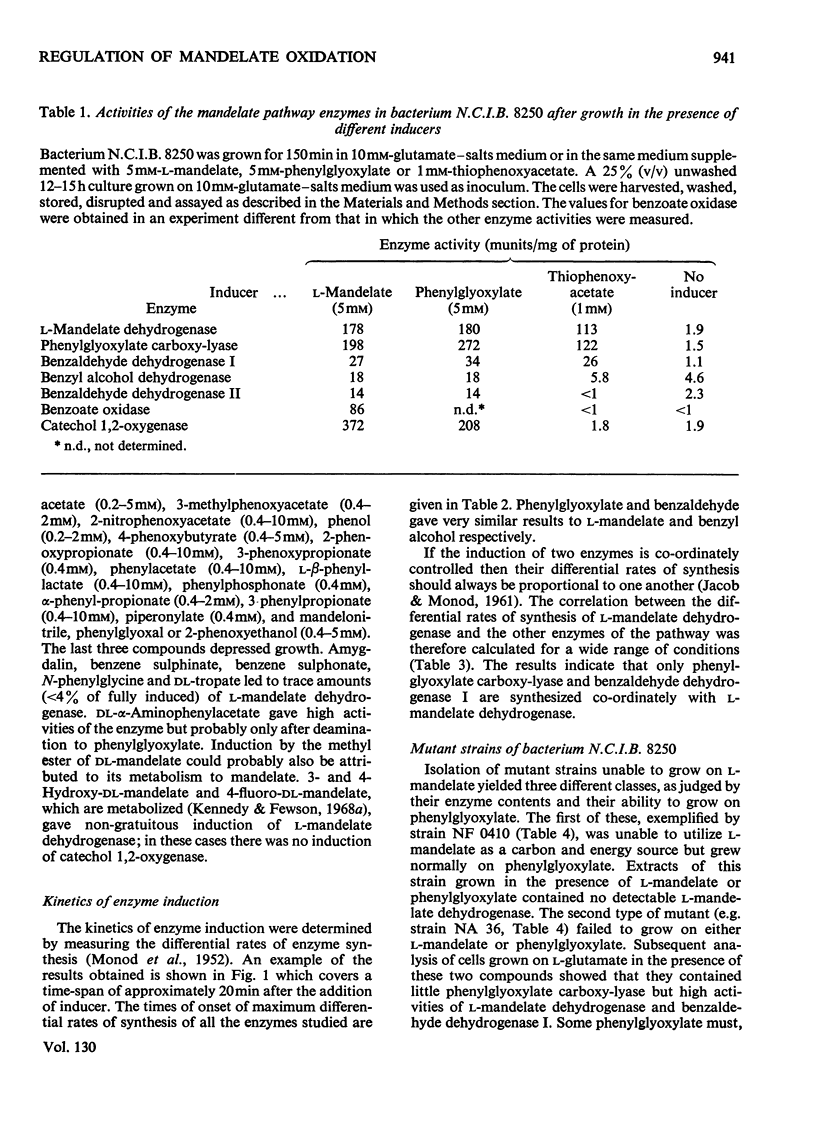
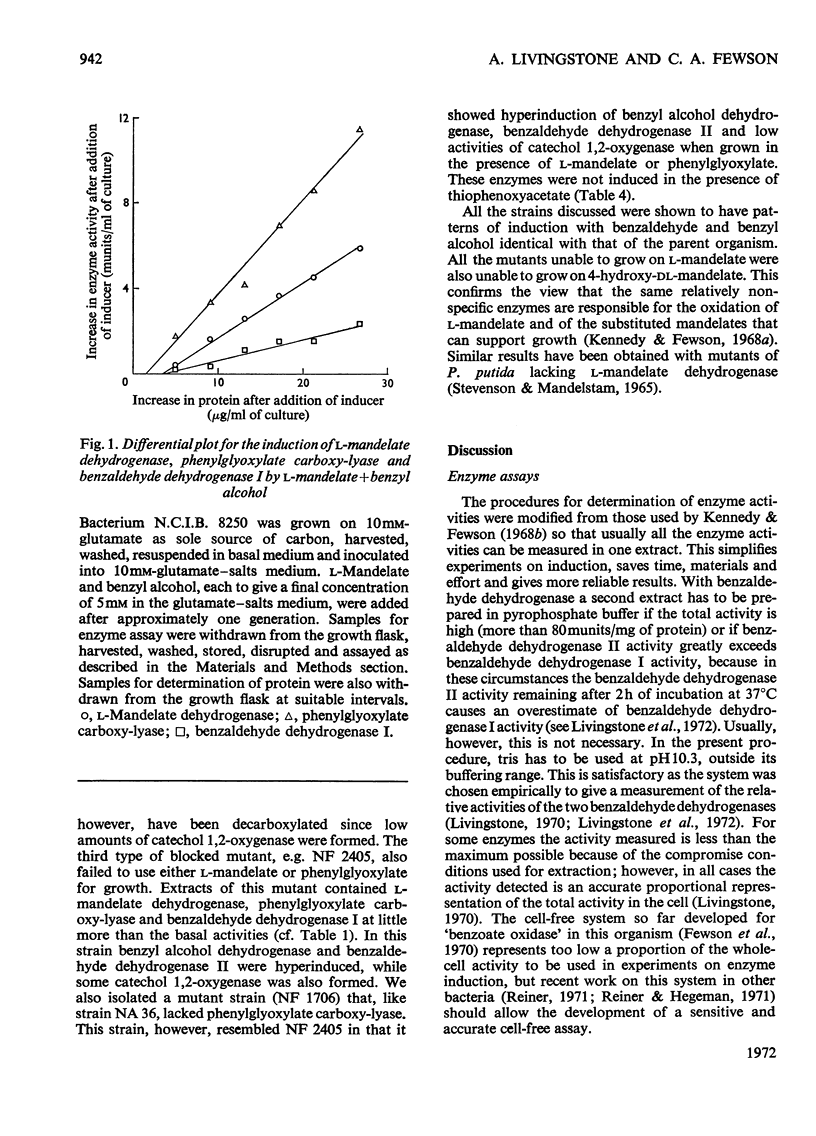
l-Mandelate is oxidized to benzoate by the enzymes l-mandelate dehydrogenase, phenylglyoxylate carboxy-lyase and benzaldehyde dehydrogenase I. Conditions have been established for measuring these three enzymes as well as benzyl alcohol dehydrogenase, benzaldehyde dehydrogenase II and catechol 1,2-oxygenase in a single cell-free extract prepared from bacterium N.C.I.B. 8250. The kinetics of induction of all these enzymes have been measured under a variety of conditions. l-Mandelate dehydrogenase, phenylglyoxylate carboxy-lyase and benzaldehyde dehydrogenase I appear to be co-ordinately regulated because (a) their differential rates of synthesis are proportional to one another under various conditions of induction and repression, (b) they are specifically and gratuitously induced by thiophenoxyacetate and a number of other compounds, and (c) mutant strains have been isolated that lack all three enzymes. Phenylglyoxylate is the primary inducer of the regulon as mutant strains lacking phenylglyoxylate carboxy-lyase form the other two enzymes in the presence of l-mandelate or phenylglyoxylate, whereas in mutant strains devoid of l-mandelate dehydrogenase activity only phenylglyoxylate induces phenylglyoxylate carboxy-lyase and benzaldehyde dehydrogenase I.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore M. A., Quayle J. R. Microbial growth on oxalate by a route not involving glyoxylate carboligase. Biochem J. 1970 Jun;118(1):53–59. doi: 10.1042/bj1180053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus I. C. Transduction and genetic homology between Pseudomonas species putida and aeruginosa. J Bacteriol. 1970 Sep;103(3):830–832. doi: 10.1128/jb.103.3.830-832.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- Fewson C. A. The growth and metabolic versatility of the gram-negative Bacterium NCIB 8250 ("Vibrio 01"). J Gen Microbiol. 1967 Feb;46(2):255–266. doi: 10.1099/00221287-46-2-255. [DOI] [PubMed] [Google Scholar]
- Harvey N. L., Fewson C. A., Holms W. H. Apparatus for batch culture of micro-organisms. Lab Pract. 1968 Oct;17(10):1134–1136. [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. 3. Isolation and properties of constitutive mutants. J Bacteriol. 1966 Mar;91(3):1161–1167. doi: 10.1128/jb.91.3.1161-1167.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. II. Isolation and properties of blocked mutants. J Bacteriol. 1966 Mar;91(3):1155–1160. doi: 10.1128/jb.91.3.1155-1160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Mandelstam J. Regulation of pathways degrading aromatic substrates in Pseudomonas putida. Enzymic response to binary mixtures of substrates. Biochem J. 1972 Feb;126(4):901–916. doi: 10.1042/bj1260901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K. Regulation of synthesis of early enzymes of p-hydroxybenzoate pathway in Pseudomonas putida. J Biol Chem. 1970 Oct 25;245(20):5304–5308. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M., Rao P. V., Vaidyanathan C. S. Involvement of the protocatechuate pathway in the metabolism of mandelic acid by Aspergillus niger. J Bacteriol. 1970 Mar;101(3):786–793. doi: 10.1128/jb.101.3.786-793.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Metabolism of mandelate and related compounds by bacterium NCIB 8250. J Gen Microbiol. 1968 Sep;53(2):259–273. doi: 10.1099/00221287-53-2-259. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone A., Fewson C. A., Kennedy S. I., Zatman L. J. Two benzaldehyde dehydrogenases in bacterium N.C.I.B. 8250. Distinguishing properties and regulation. Biochem J. 1972 Dec;130(4):927–935. doi: 10.1042/bj1300927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSETT T. COOLING DEVICE FOR USE WITH A SONIC OSCILLATOR. Appl Microbiol. 1965 Mar;13:254–256. doi: 10.1128/am.13.2.254-256.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M., Hegeman G. D. Metabolism of benzoic acid by bacteria. Accumulation of (-)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by mutant strain of Alcaligenes eutrophus. Biochemistry. 1971 Jun 22;10(13):2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of benzoic acid by bacteria: 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid is an intermediate in the formation of catechol. J Bacteriol. 1971 Oct;108(1):89–94. doi: 10.1128/jb.108.1.89-94.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. L., Hegeman G. D. Clustering of functionally related genes in Pseudomonas aeruginosa. J Bacteriol. 1969 Jul;99(1):353–355. doi: 10.1128/jb.99.1.353-355.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. L., Hegeman G. D. Genetics of the mandelate pathway in Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1270–1276. doi: 10.1128/jb.108.3.1270-1276.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. L. Regulation of the mandelate pathway in Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1257–1269. doi: 10.1128/jb.108.3.1257-1269.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., GUNSALUS I. C., GUNSALUS C. F. The enzymatic conversion of mandelic acid to benzoic acid. II. Properties of the particulate fractions. J Bacteriol. 1953 Nov;66(5):543–547. doi: 10.1128/jb.66.5.543-547.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. The Oxidation of Aromatic Compounds by Fluorescent Pseudomonads. J Bacteriol. 1948 Apr;55(4):477–494. doi: 10.1128/jb.55.4.477-494.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I. L., Mandelstam J. Induction and multi-sensitive end-product repression in two converging pathways degrading aromatic substances in Pseudomonas fluorescens. Biochem J. 1965 Aug;96(2):354–362. doi: 10.1042/bj0960354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véron M. Taxonomie numériques de vibrions et de certaines bactéries comparables. II. Corrélation entre les similitudes phénétiques et la composition en bases de l'ADN. Ann Inst Pasteur (Paris) 1966 Dec;111(6):671–709. [PubMed] [Google Scholar]
- Wheelis M. L., Stanier R. Y. The genetic control of dissimilatory pathways in Pseudomonas putida. Genetics. 1970 Oct;66(2):245–266. doi: 10.1093/genetics/66.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]


