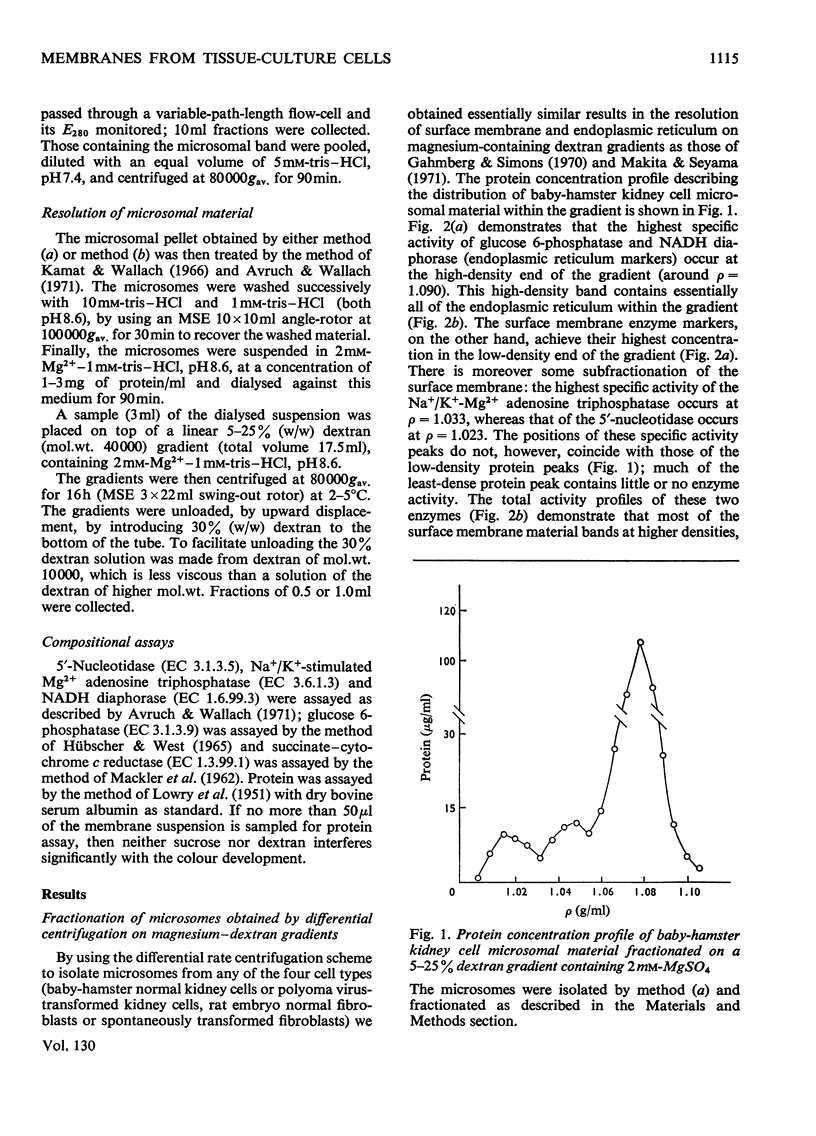
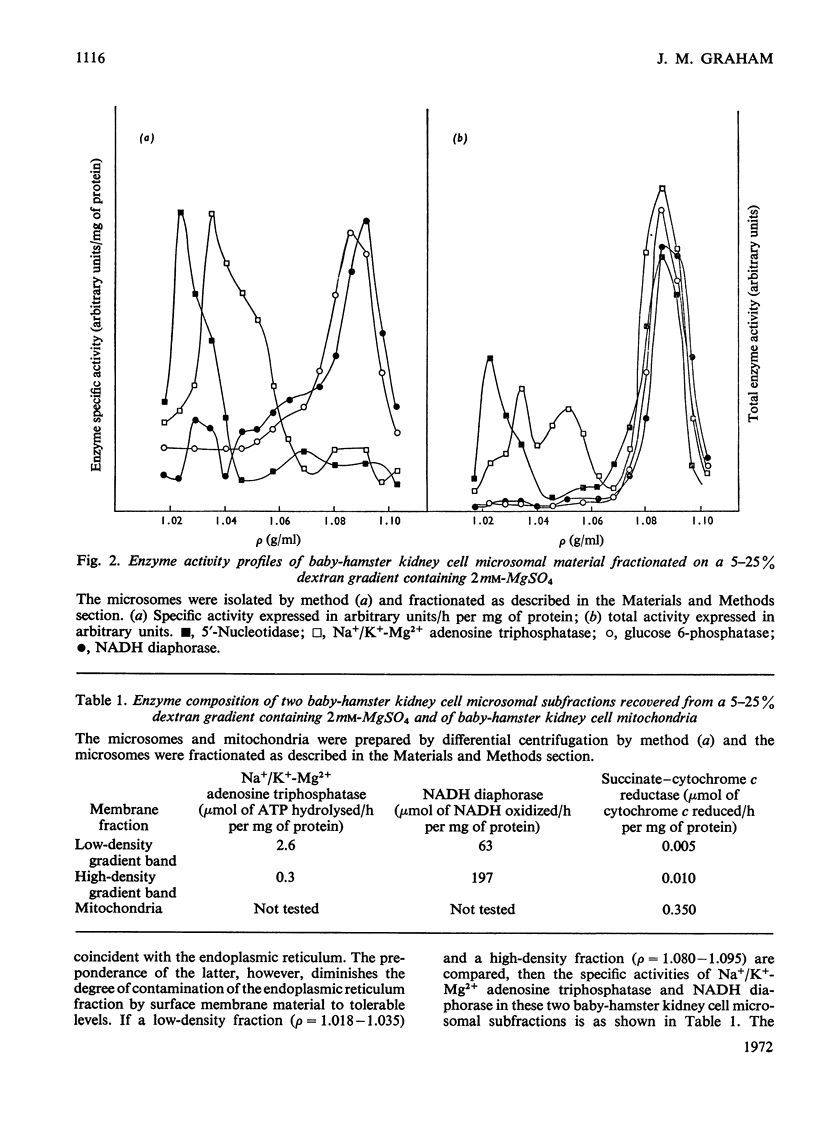
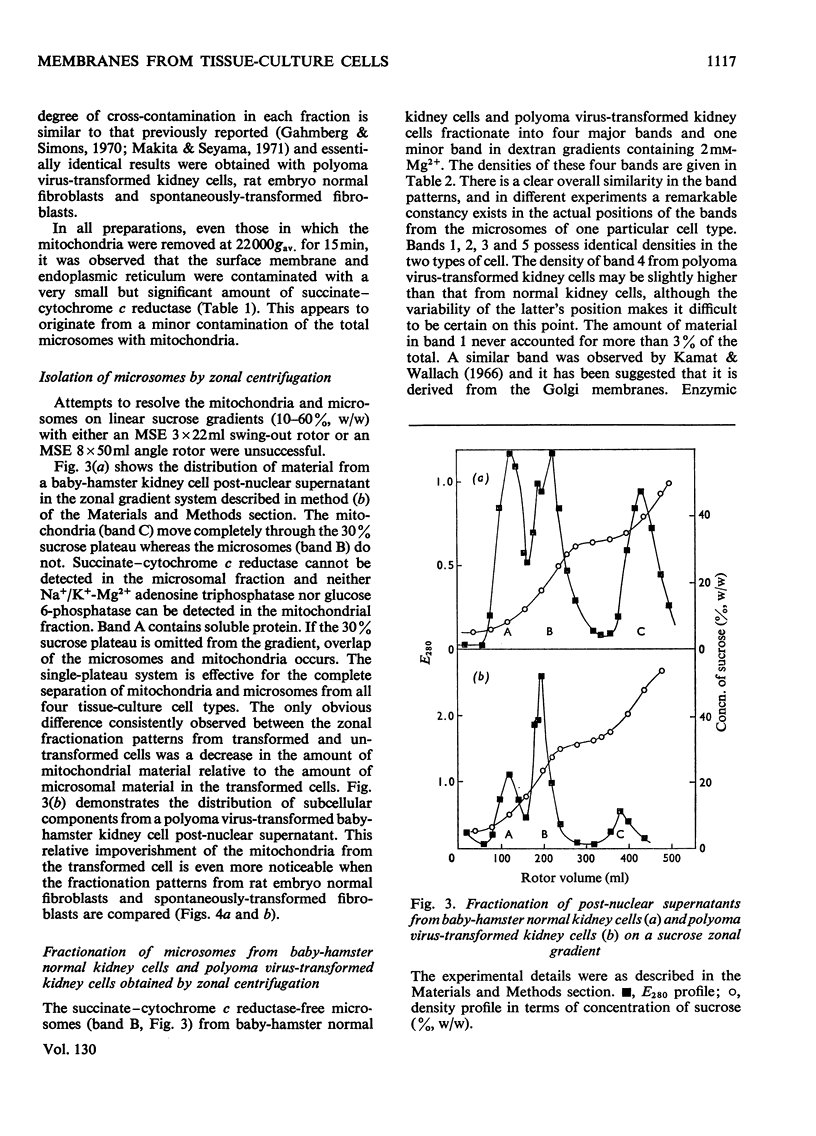
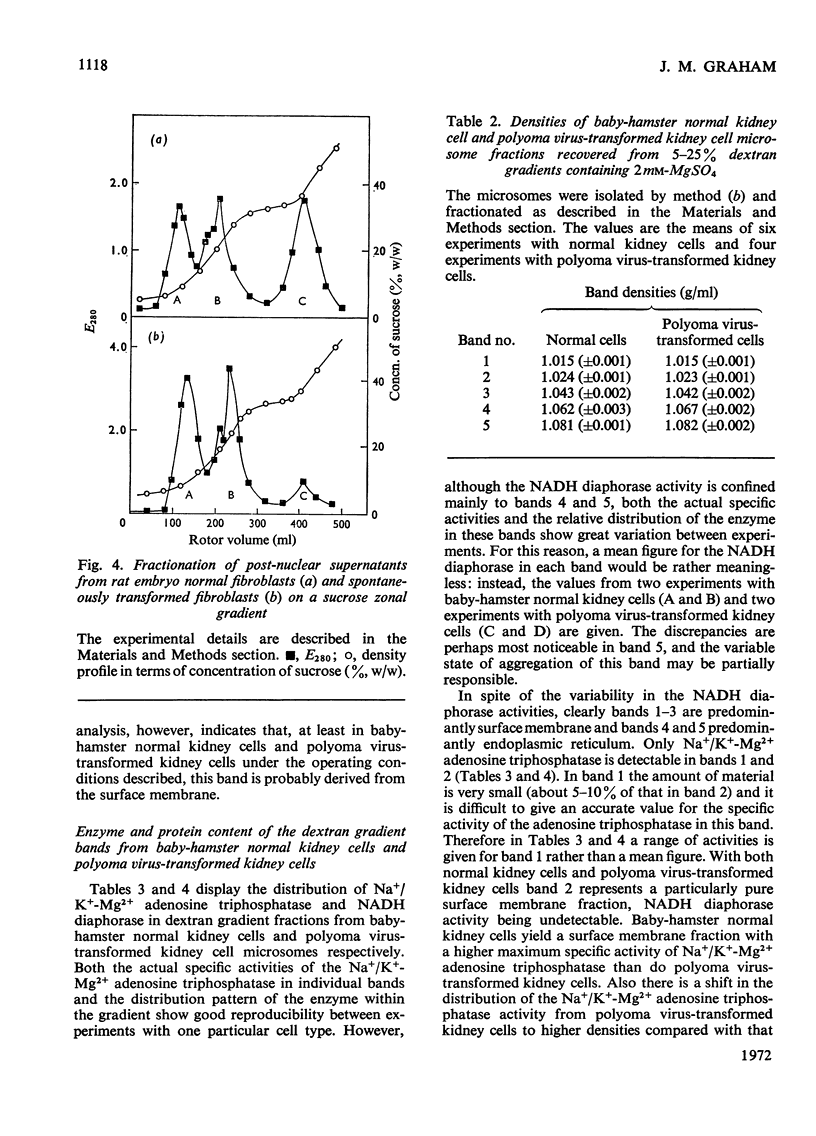
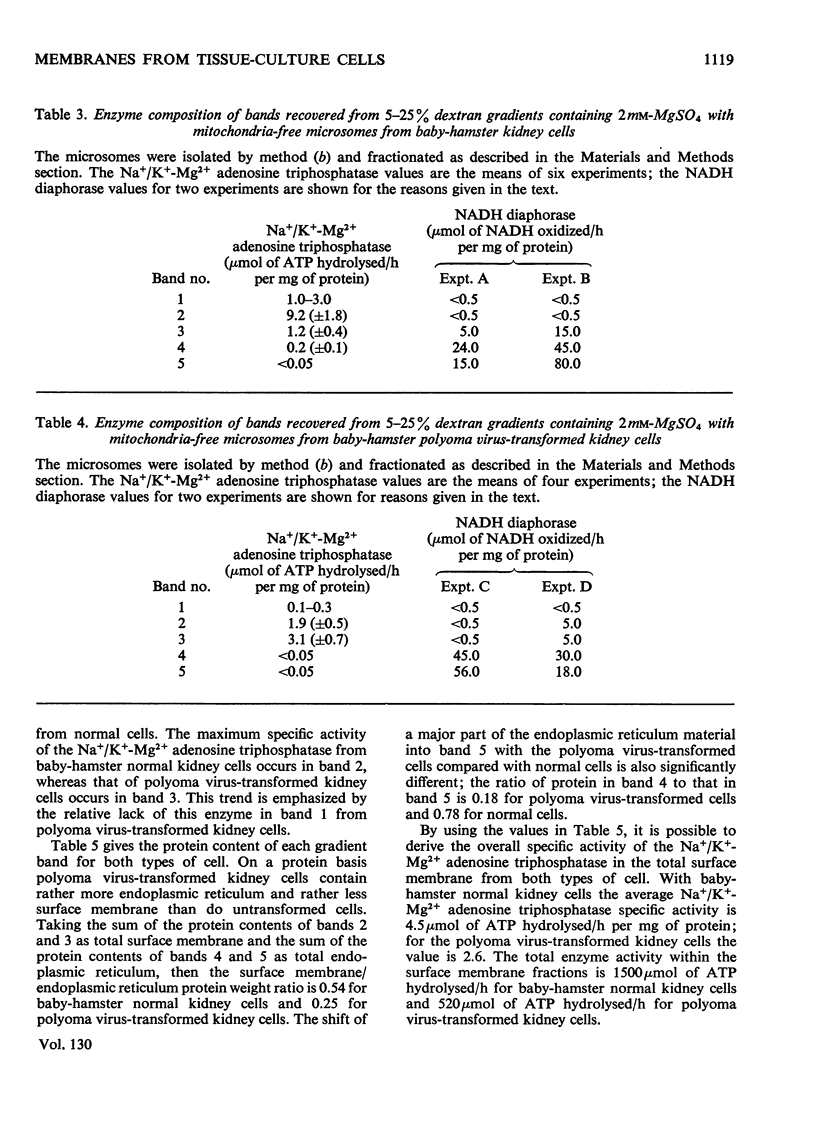
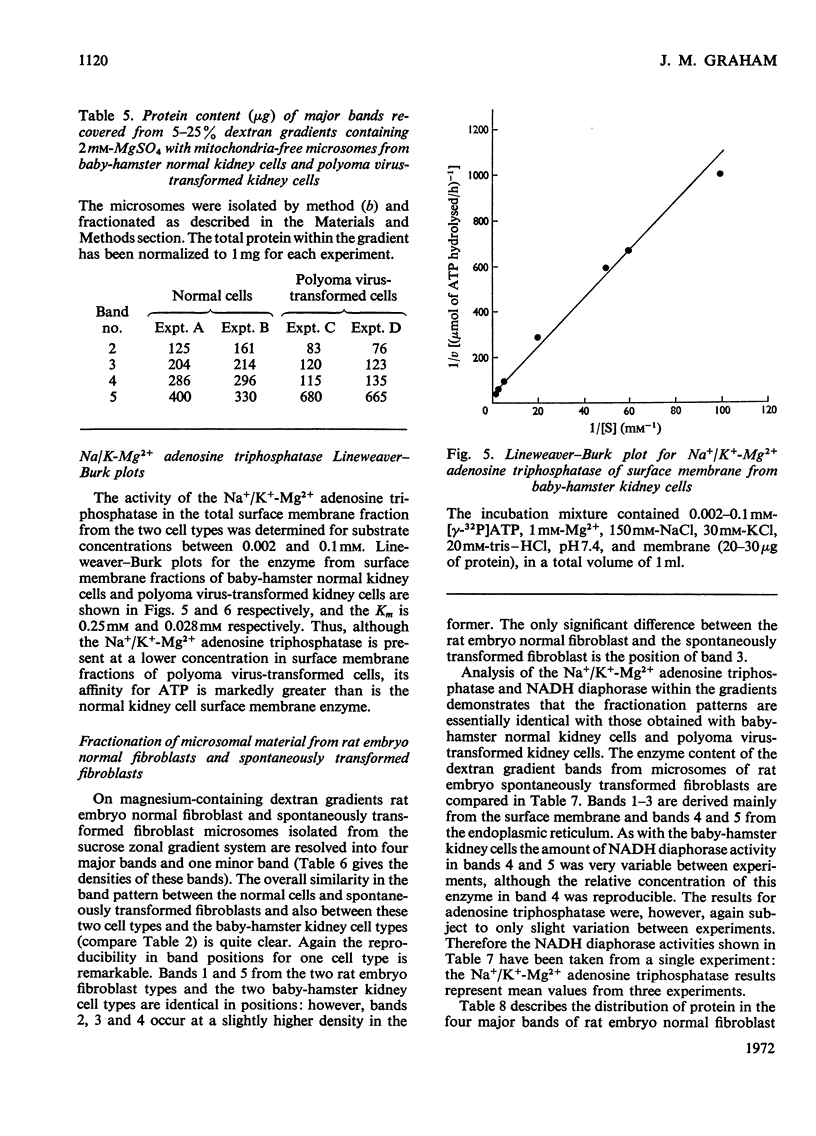
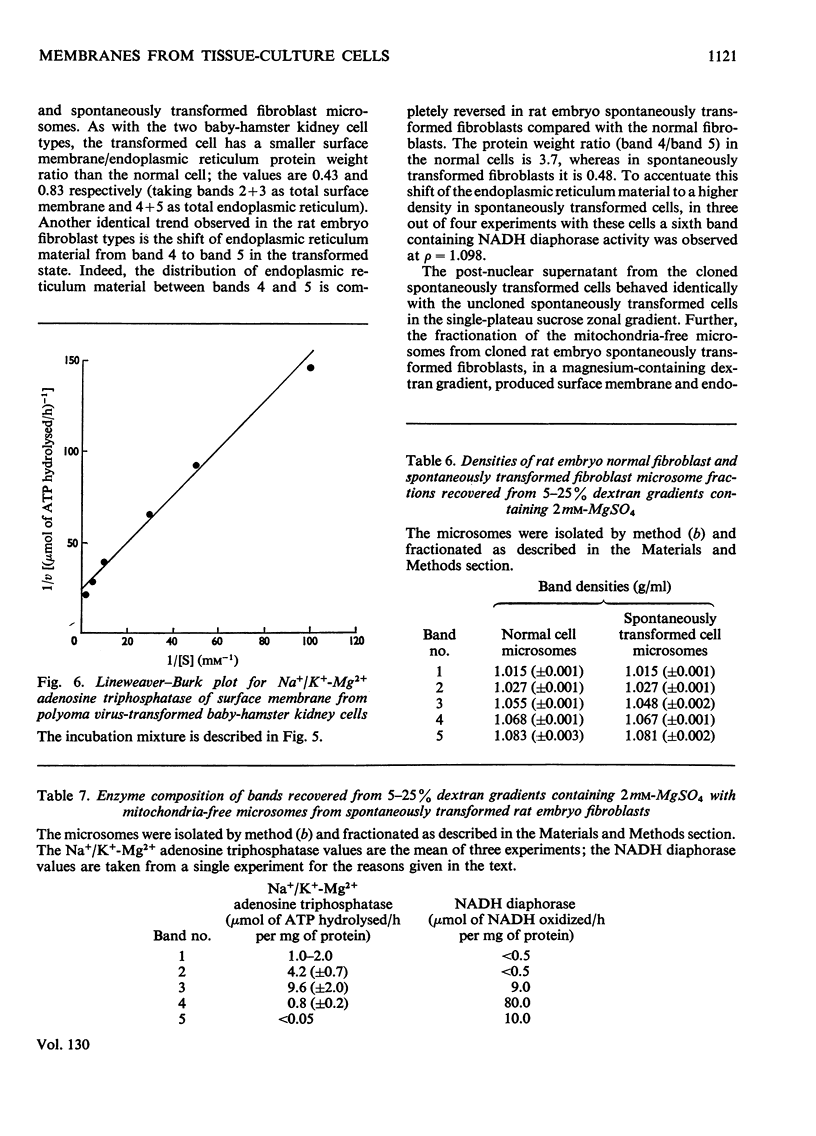
Abstract
Homogenates of baby-hamster kidney cells and rat embryo fibroblasts prepared by nitrogen cavitation contain a small population of slowly sedimenting mitochondria or mitochondrial fragments, which contaminate the microsomal fraction. This appears to limit the resolution of surface membrane and endoplasmic reticulum on magnesium-containing dextran gradients. The microsomal material and mitochondria can, however, be completely separated on a 10–60% (w/w) sucrose zonal gradient containing a 30% sucrose plateau. On magnesium-containing dextran gradients this mitochondria-free microsomal material can be resolved into at least two surface membrane fractions and at least two endoplasmic reticulum fractions. Comparison of polyoma virus-transformed and normal baby-hamster kidney cells reveals some interesting differences in their microsomal fractionation patterns and the characteristics of the Na+/K+-Mg2+ adenosine triphosphatase of their surface membranes, in particular a tenfold lower Km in the virus-transformed cells. The fractionation patterns of normal and spontaneously transformed rat embryo fibroblasts are also briefly discussed, particularly in relation to the significance of the observation that both the surface membrane and endoplasmic reticulum from these cells can be subfractionated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bergelson L. D., Dyatlovitskaya E. V., Torkhovskaya T. I., Sorokina I. B., Gorkova N. P. Phospholipid composition of membranes in the tumor cell. Biochim Biophys Acta. 1970 Jul 14;210(2):287–298. doi: 10.1016/0005-2760(70)90173-6. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Warmsley A. M., Pasternak C. A. Phospholipid synthesis and degradation during the life-cycle of P815Y mast cells synchronized with excess of thymidine. Biochem J. 1970 Sep;119(3):489–492. doi: 10.1042/bj1190489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- DAS M. L., HAAK E. D., CRANE F. L. PROTEOLIPIDS. IV. FORMATION OF COMPLEXES BETWEEN CYTOCHROME C AND PURIFIED PHOSPHOLIPIDS. Biochemistry. 1965 May;4:859–865. doi: 10.1021/bi00881a010. [DOI] [PubMed] [Google Scholar]
- Dijong I., Mora P. T., Brady R. O. Gas chromatographic determination of gangliosides in mouse cell lines and in virally transformed derivative lines. Biochemistry. 1971 Oct 26;10(22):4039–4044. doi: 10.1021/bi00798a005. [DOI] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. V. On the lipid dependence of some phosphohydrolases of isolated rat-liver plasma membranes. Biochim Biophys Acta. 1968 Apr 29;150(3):341–353. doi: 10.1016/0005-2736(68)90133-8. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Simons K. Isolation of plasma membrane fragments from BHK21 cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(2):176–182. doi: 10.1111/j.1699-0463.1970.tb04284.x. [DOI] [PubMed] [Google Scholar]
- Glaser M., Simpkins H., Singer S. J., Sheetz M., Chan S. I. On the interactions of lipids and proteins in the red blood cell membrane. Proc Natl Acad Sci U S A. 1970 Mar;65(3):721–728. doi: 10.1073/pnas.65.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. M., Higgins J. A., Green C. The isolation of rat liver plasma membrane fragments. Biochim Biophys Acta. 1968 Mar 1;150(2):303–305. doi: 10.1016/0005-2736(68)90173-9. [DOI] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Murakami W. T. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Jan;59(1):254–261. doi: 10.1073/pnas.59.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haris R. A., Asbell M. A., Green D. E. Correlation between light-scattering changes and the en ergy state of phosphorylating submitochondrial particles. Arch Biochem Biophys. 1969 Apr;131(1):316–318. doi: 10.1016/0003-9861(69)90137-4. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Inbar M., Vlodavsky I., Sachs L. Availability of L-fucose-like sites on the surface membrane of normal and transformed mammalian cells. Biochim Biophys Acta. 1972 Feb 11;255(2):703–708. doi: 10.1016/0005-2736(72)90175-7. [DOI] [PubMed] [Google Scholar]
- KAMAT V. B., WALLACH D. F. SEPARATION AND PARTIAL PURIFICATION OF PLASMA-MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA MICROSOMES. Science. 1965 Jun 4;148(3675):1343–1345. doi: 10.1126/science.148.3675.1343. [DOI] [PubMed] [Google Scholar]
- Kryukova I. N., Babkova O. V., Obukh I. B. Malignant and transforming activity of Rous sarcoma virus (RSV). 3. Detection of tumor-specific transplantation and membrane antigens in mouse cell lines transformed in vitro by RSV. J Natl Cancer Inst. 1971 Oct;47(4):819–827. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb J. N., Kimberg D. V. Sedimentation properties of rat liver mitochondria. Effects of cortisone treatment. J Cell Biol. 1970 Jul;46(1):17–26. doi: 10.1083/jcb.46.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita A., Seyama Y. Alterations of Forssman-antigenic reactivity and of monosaccharide composition in plasma membrane from polyoma-transformed hamster cells. Biochim Biophys Acta. 1971 Aug 13;241(2):403–411. doi: 10.1016/0005-2736(71)90040-x. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Brady R. O., Bradley R. M., McFarland V. W. Gangliosides in DNA virus-transformed and spontaneously transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1290–1296. doi: 10.1073/pnas.63.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peery C. V., Johnson G. S., Pastan I. Adenyl cyclase in normal and transformed fibroblasts in tissue culture. Activation by prostaglandins. J Biol Chem. 1971 Sep 25;246(18):5785–5790. [PubMed] [Google Scholar]
- Perdue J. A., Kletzien R., Miller K., Pridmore G., Wray V. L. The isolation and characterization of plasma membranes from cultured cells. II. The chemical composition of membrane isolated from uninfected and oncogenic RNA virus-converted parenchyma-like cells. Biochim Biophys Acta. 1971 Dec 3;249(2):435–453. doi: 10.1016/0005-2736(71)90121-0. [DOI] [PubMed] [Google Scholar]
- Perdue J. F., Kletzien R., Miller K. The isolation and characterization of plasma membrane from cultured cells. I. The chemical composition of membrane isolated from uninfected and oncogenic RNA virus-converted chick embryo fibroblasts. Biochim Biophys Acta. 1971 Dec 3;249(2):419–434. doi: 10.1016/0005-2736(71)90120-9. [DOI] [PubMed] [Google Scholar]
- Perdue J. F., Sneider J. The isolation and characterization of the plasma membrane from chick embryo fibroblasts. Biochim Biophys Acta. 1970;196(2):125–140. doi: 10.1016/0005-2736(70)90001-5. [DOI] [PubMed] [Google Scholar]
- Renkonen O., Gahmberg C. G., Simons K., Käriäinen L. The lipids of the plasma membranes and endoplasmic reticulum from cultured baby hamster kidney cells (BHK21). Biochim Biophys Acta. 1972 Jan 17;255(1):66–78. doi: 10.1016/0005-2736(72)90008-9. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick R. W., Tollaksen S. L., Nance S. L., Thomson J. F. The unique distribution of ornithine aminotransferase in rat liver mitochondria. Arch Biochem Biophys. 1970 Jan;136(1):212–218. doi: 10.1016/0003-9861(70)90343-7. [DOI] [PubMed] [Google Scholar]
- Tanaka R., Sakamoto T. Molecular structure in phospholipid essential to activate (Na+ K+ Mg2+)-dependent ATPase and (K+ Mg2+)-dependent phosphatase of bovine cerebral cortex. Biochim Biophys Acta. 1969;193(2):384–393. doi: 10.1016/0005-2736(69)90198-9. [DOI] [PubMed] [Google Scholar]
- WALLACH D. F., KAMAT V. B. PLASMA AND CYTOPLASMIC MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA. Proc Natl Acad Sci U S A. 1964 Sep;52:721–728. doi: 10.1073/pnas.52.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F., Gordon A. Lipid protein interactions in cellular membranes. Fed Proc. 1968 Nov-Dec;27(6):1263–1268. [PubMed] [Google Scholar]
- Wheeler K. P., Whittam R. ATPase activity of the sodium pump needs phosphatidylserine. Nature. 1970 Jan 31;225(5231):449–450. doi: 10.1038/225449a0. [DOI] [PubMed] [Google Scholar]


