Abstract
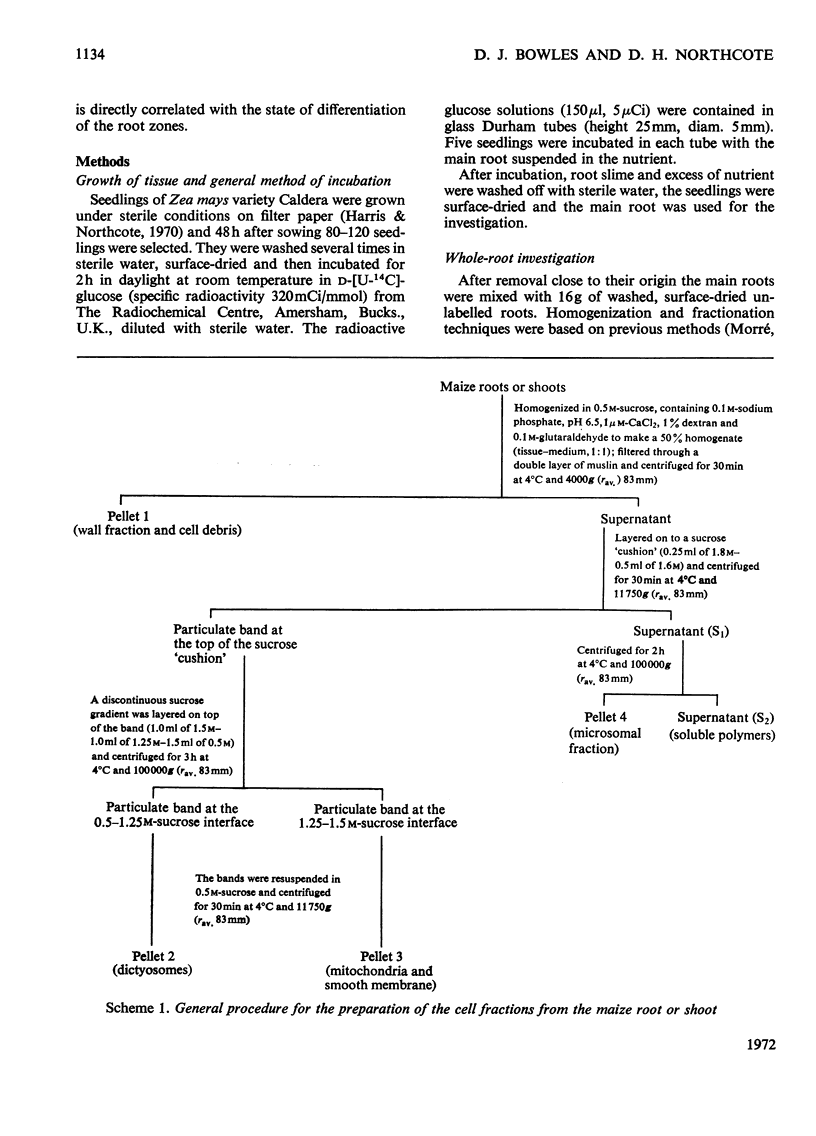
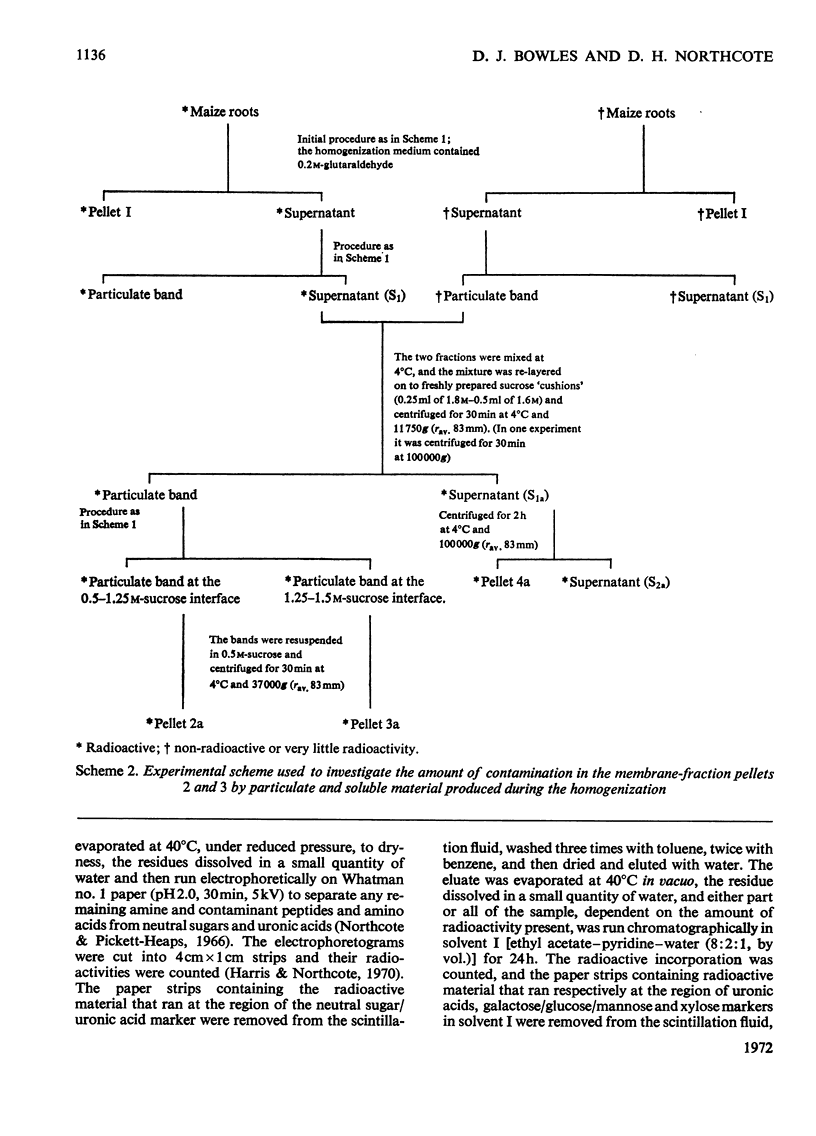
1. Subcellular fractionation of maize roots resulted in the isolation of the following enriched fractions: cell wall, dictyosome, smooth-membrane and rough-microsomal fractions. In addition, extracellular polysaccharide of the root slime was isolated. 2. Maizeseedling roots were incubated in vivo with d-[U-14C]glucose, and the pattern of incorporation of radioactivity into the polysaccharides of each fraction was investigated. 3. The differentiation of maize-root cells with respect to the synthesis of specific extracellular polysaccharide directly relates to the polysaccharide synthesized and transported within the membrane system of the cell. A fucose-containing polysaccharide, characteristic only of root slime, was present only in the membrane system of the root-tip region of the root. Regions of typical secondary wall development within the root were characterized by an increased incorporation of radioactivity into xylose of polysaccharide within the membrane system. 4. The incorporation of radioactivity into glucan polymers in the membrane fractions was very low in all regions of the root. Since in regions of secondary wall development greater than 60% of all radioactive incorporation was into a glucan polymer, it can be inferred that this polymer, most probably cellulose, is not synthesized or transported within the compartments of the membrane system. It is suggested that synthesis of cellulose occurs at the surface of the plasmalemma. 5. Maize-root cells contained 40 times more rough endoplasmic reticulum than dictyosome membrane. The relative specific radioactivities of each fraction indicated that polysaccharide was concentrated in the region of the Golgi apparatus, which showed a 100% increase in specific radioactivity compared with the rough endoplasmic reticulum. The Golgi apparatus can thus be regarded as a localized focal point on the synthetic and transport system of polysaccharide by the intracellular membrane compartments.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beams H. W., Kessel R. G. The Golgi apparatus: structure and function. Int Rev Cytol. 1968;23:209–276. doi: 10.1016/s0074-7696(08)60273-9. [DOI] [PubMed] [Google Scholar]
- Bennett G., Leblond C. P. Formation of cell coat material for the whole surface of columnar cells in the rat small intestine, as visualized by radioautography with L-fucose-3H. J Cell Biol. 1970 Aug;46(2):409–416. doi: 10.1083/jcb.46.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W. P., Morré D. J., Mollenhauer H. H. Structure of isolated plant Golgi apparatus revealed by negative staining. J Cell Biol. 1966 Feb;28(2):169–179. doi: 10.1083/jcb.28.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Polysaccharide formation in plant Golgi bodies. Biochim Biophys Acta. 1971 Apr 20;237(1):56–64. doi: 10.1016/0304-4165(71)90029-8. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Northcote D. H. Nematode-induced syncytium--a multinucleate transfer cell. J Cell Sci. 1972 May;10(3):789–809. doi: 10.1242/jcs.10.3.789. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J. Phospholipid class and fatty acid composition of golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry. 1970 Jan 6;9(1):19–25. doi: 10.1021/bi00803a003. [DOI] [PubMed] [Google Scholar]
- Morré D. J. In vivo incorporation of radioactive metabolites by Golgi apparatus and other cell fractions of onion stem. Plant Physiol. 1970 Jun;45(6):791–799. doi: 10.1104/pp.45.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote D. H. Fine structure of cytoplasm in relation to synthesis and secretion in plant cells. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):21–30. doi: 10.1098/rspb.1969.0033. [DOI] [PubMed] [Google Scholar]
- Northcote D. H., Lewis D. R. Freeze-etched surfaces of membranes and organelles in the cells of pea root tips. J Cell Sci. 1968 Jun;3(2):199–206. doi: 10.1242/jcs.3.2.199. [DOI] [PubMed] [Google Scholar]
- Northcote D. H. Organization of structure, synthesis and transport within the plant during cell division and growth. Symp Soc Exp Biol. 1971;25:51–69. [PubMed] [Google Scholar]
- Northcote D. H., Pickett-Heaps J. D. A function of the Golgi apparatus in polysaccharide synthesis and transport in the root-cap cells of wheat. Biochem J. 1966 Jan;98(1):159–167. doi: 10.1042/bj0980159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K., Northcote D. H. The structure of sycamore callus cells during division in a partially synchronized suspension culture. J Cell Sci. 1970 Mar;6(2):299–321. doi: 10.1242/jcs.6.2.299. [DOI] [PubMed] [Google Scholar]
- Roberts R. M., Butt V. S. Patterns of cellulose synthesis in maize root-tips. A chemical and autoradiographic study. Exp Cell Res. 1967 Jun;46(3):495–510. doi: 10.1016/0014-4827(67)90376-x. [DOI] [PubMed] [Google Scholar]
- Staehelin A. Die Ultrastruktur der Zellwand und des Chloroplasten von Chlorella. Z Zellforsch Mikrosk Anat. 1966;74(3):325–350. [PubMed] [Google Scholar]
- Stoddart R. W., Northcote D. H. Metabolic relationships of the isolated fractions of the pectic substances of actively growing sycamore cells. Biochem J. 1967 Oct;105(1):45–59. doi: 10.1042/bj1050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. II. Carbohydrate constituents of each main component. Biochem J. 1961 Dec;81:455–464. doi: 10.1042/bj0810455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Cynkin M. A. Glycoprotein biosynthesis. Incorporation of glycosyl groups into endogenous acceptors in a Golgi apparatus-rich fraction of liver. J Biol Chem. 1971 Jan 10;246(1):143–151. [PubMed] [Google Scholar]
- Whaley W. G., Dauwalder M., Kephart J. E. The Golgi apparatus and an early stage in cell plate formation. J Ultrastruct Res. 1966 Apr;15(1):169–180. doi: 10.1016/s0022-5320(66)80102-8. [DOI] [PubMed] [Google Scholar]
- Wooding F. B. Radioautographic and chemical studies of incorporation into sycamore vascular tissue walls. J Cell Sci. 1968 Mar;3(1):71–80. doi: 10.1242/jcs.3.1.71. [DOI] [PubMed] [Google Scholar]
- Zagury D., Uhr J. W., Jamieson J. D., Palade G. E. Immunoglobulin synthesis and secretion. II. Radioautographic studies of sites of addition of carbohydrate moieties and intracellular transport. J Cell Biol. 1970 Jul;46(1):52–63. doi: 10.1083/jcb.46.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]