Abstract
Background
We examined the effect of hepatitis C virus (HCV) seropositivity on risk of death among people receiving their first antiretroviral treatment (ART) for HIV infection.
Methods
In British Columbia, the HIV/ AIDS Drug Treatment Program is the only source of free ART. Patients who initiated a triple-drug ART regimen between July 31, 1996, and July 31, 2000, were included if they were ART-naive and had baseline HCV serological data. Outcomes of interest for survival analysis were deaths from natural and HIV-related causes, with a data cutoff of June 30, 2003.
Results
Of 1186 eligible subjects, 606 (51%) were HCV positive and 580, negative. Fewer HCV-positive people were male (78% v. 93%, p < 0.001) and had an AIDS diagnosis at baseline (11% v. 15%, p = 0.028). Their CD4 fraction was significantly higher at baseline (19% v. 16% of T lymphocytes, p < 0.001) but their absolute CD4 counts, log HIV viral load and the type of ART initiated were similar to those of HCV negative people. Of 163 deaths (from natural causes only) during the study period, 118 (19%) were in HCV positive and 45 (8%) in HCV negative patients (p < 0.001); of the 114 deaths attributed to HIV infection, these proportions were 79 (13%) versus 35 (6%; p < 0.001). After adjustment for potential confounders, HCV seropositivity remained predictive of death (adjusted hazard ratio [HR] 2.20, 95% confidence interval [CI] 1.50– 3.21, p < 0.001), especially HIV-related death (adjusted HR 1.75, 95% CI 1.13– 2.72, p = 0.012).
Interpretation
In this population-based HIV treatment program, we found HCV seropositivity to be an independent predictor of mortality, especially death related to HIV infection.
Seropositivity for hepatitis C virus (HCV) is prevalent among people who are HIV-positive.1 Although several authors have found that liver disease has become a leading cause of death among those infected with HIV,2,3,4 debate continues as to the effect of HCV infection on HIV disease progression, as measured by new AIDS-defining illnesses, CD4 T-cell decline or HIV-related mortality.4,5,6,7,8,9 Mortality in this population can be strongly confounded by factors such as adherence to a course of antiretroviral therapy (ART), illicit use of injected drugs and previous administration of ART.
During the 1990s, Vancouver experienced an explosive epidemic of HIV and HCV infection among the city's 10 000 users of injected drugs;10 currently, more than 30% are coinfected with both viruses.11 Here we report on the effect of HCV serostatus on the risk of death among participants in a population-based HIV and AIDS treatment program who had received no previous ART, adjusting for adherence to ART and history of injection drug use. Specifically, we describe the effect of HCV serostatus on risk of death, particularly HIV-related death.
Methods
The HIV/AIDS Drug Treatment Program, described in detail elsewhere,12 is the only source of free antiretroviral drugs in British Columbia. The program follows therapeutic guidelines that are consistent with international standards.13 Data for this analysis were drawn from all program clients who started their first ART between July 31, 1996 and July 31, 2000 and began it with a triple-drug regimen consisting of 2 nucleoside analogue reverse-transcriptase inhibitors (NRTIs) plus either a protease inhibitor (PI) or a non-nucleoside reverse-transcriptase inhibitor (NNRTI) (n = 1388). The analysis was further restricted to those for whom HCV serologic data were available (n = 1186). Our cutoff date for follow-up data was June 30, 2003.
Mortality data were obtained from British Columbia Vital Statistics. We used codes from the International Classification of Diseases, tenth revision,14,15 to evaluate the underlying cause of death; the specific codes used16 can be found in an online appendix (available at www.cmaj.ca/cgi/content/full/173/2/160/DC1). HCV serologic data were obtained from plasma samples stored within 6 months before the initiation of ART; details of the assay method can also be found in the online appendix.
Approval for this study was obtained from the research ethics board of the University of British Columbia.
Two outcome measures were important to our analysis: death from natural biological causes (i.e., not caused by an accident, overdose, suicide or murder; for the purposes of this study, death from such causes was classified as a non-event), and death specifically attributed to HIV infection. As stated, we analyzed data available up to June 30, 2003; participants who died of nonbiological causes in the first analysis, or of nonbiological or biological but HIV-unrelated causes in the second, stopped being counted in the analysis (i.e., were censored) from the last known date of contact with the HIV/AIDS Drug Treatment Program.
The variables considered in our analyses included clients' sex; age (in 10-year increments); history of injection drug use (used or not used); whether AIDS was diagnosed at baseline; adherence to HIV medication during the first year of therapy; changes over time from baseline in CD4 count (categorized into ≤0.09 х 109/L, 0.10–0. 19 х 109/L, 0.20–0.35 х 109/L and > 0.35 х 109/L [the reference category]); time-dependent log10 HIV viral load per log increase from the baseline measurement; and whether an NNRTI or a PI was part of the initial ART drug combination.
Adherence to ART has been validated as a strong predictor of both virologic response17 and survival.18,19 Our study subjects were considered adherent to treatment if the length of time their dispensed antiretroviral medication was expected to last was more than 95% of the patient's follow-up (or survival) time. Note that this calculation was restricted to each study patient's first year of ART in order to avoid the reverse causation that could occur among those who become too sick to take medication and therefore cease therapy.
Hypothesized interactions were tested a priori between HCV infection and any history of injection drug use, and between injection drug use and adherence to medication.
HCV treatment was not factored into the statistical model because effective HCV treatment was unavailable before June 2003, when Pegetron (peginterferon bundled with ribavirin) was approved for prescription use in British Columbia. Response rates to regular interferon treatment (with or without ribavirin) in patients coinfected with HIV and HCV are very low;16 we therefore considered the effect of HCV treatment in this population to be negligible.
In our statistical analyses we used an “intention to continue treatment” approach whereby data were included for analysis according to when the eligible study subject was first dispensed ART, regardless of whether the treatment was later discontinued or modified.
We compared data for HCV-positive and -negative study subjects by means of parametric analyses as well as methods unaffected by distribution. We used Pearson's χ2 test to analyze categorical data, Fisher's exact test for contingency tables in which one- quarter or more of the expected cell frequencies were less than 5, and the Wilcoxon rank-sum test for continuous variables. We estimated cumulative mortality rates using the Kaplan– Meier method and compared survival curves with the Wilcoxon log-rank test. Finally, we calculated unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) with Cox proportional-hazards methods. Potential confounding factors adjusted for in the analysis included age, sex, type of ART regimen, time-updated CD4 count and viral load, injection drug use and adherence to treatment. Time was measured in months from the start of antiretroviral treatment. We validated the assumption of proportional hazards by inspecting plots of the log of the cumulative hazard functions in the HCV positive and HCV negative study groups. Data were right-censored at the time of the event of interest, either at June 30, 2003, or at the date of the last preceding contact with the HIV/ AIDS Drug Treatment Program.
Results
There were 1186 people whose data were included for analysis: 606 (51%) were HCV positive and 580 (49%) HCV negative. Their characteristics at baseline are summarized in Table 1. Compared with the HCV negative subjects, those who were HCV positive were less likely to be male (78% v. 93%, p < 0.001) and to have had an AIDS diagnosis at baseline (11% v. 15%, p = 0.028); they also had a lower median CD4 fraction (0.19 v. 0.16, p < 0.001). The 2 groups were statistically similar for absolute baseline CD4 count, baseline log HIV viral load and whether their ART was initiated with an NNRTI or a PI.
Table 1
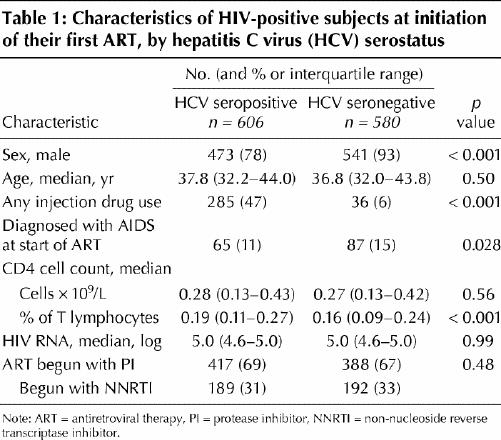
The median follow-up for all study subjects was 52.3 months (interquartile range [IQR] 39.5– 66.7 mo). Among HCV positive clients, the median follow-up was 49.9 (IQR 36.8– 63.9) months, compared with 54.8 (IQR 42.7– 70.1) months among HCV negative subjects. Of 163 deaths from natural causes that occurred during our study, 118 (72%) were among HCV positive patients and 45 (28%), HCV negative patients (p < 0.001). There were also 45 deaths unrelated to physical health, including 38 HCV positive clients (6% of that population) and 7 HCV negative (1%).
People coinfected with HIV and HCV were significantly more likely to die of HIV-related causes than were those infected with HIV alone (13% v. 6%, p < 0.001). Of those who died of liver-related causes, 7 (1%) were HCV positive and 1 (< 1%) HCV negative; cancer-related causes, 3 HCV positive and 2 HCV negative (both < 1%); and other causes, 20 (3%) HCV positive and 7 (1%) HCV negative. Cause of death was unknown for 9 HCV positive (1.5% v. 0 HCV negative) subjects.
Fig. 1 displays the results of Kaplan–Meier analysis, in which HCV serostatus was strongly associated with time to death (p < 0.001), even after restricting the analysis to people who were more than 95% adherent to their antiretroviral medication in the first year of treatment (p < 0.001). Cumulative survival at 1, 3 and 5 years after ART initiation was as follows: HCV positive at 1 year 0.97 (95% CI 0.96– 0.98), at 3 years 0.92 (95% CI 0.90–0.94) and at 5 years 0.85 (95% CI 0.82–0.88); HCV negative at 1 year 0.98 (95% CI 0.97– 0.99), at 3 years 0.96 (95% CI 0.94–0.98) and at 5 years 0.94 (95% CI 0.92–0.96).
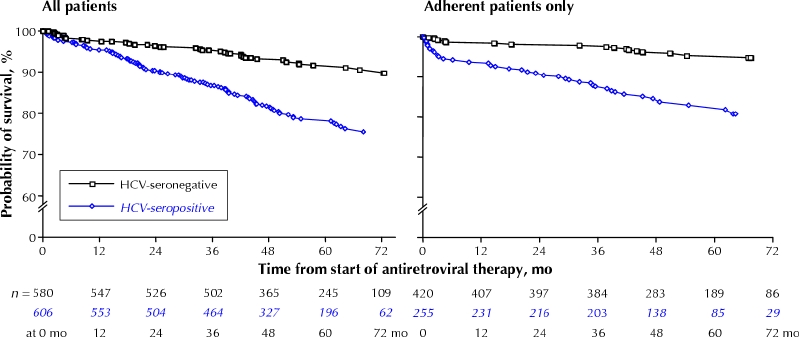
Fig. 1: Probability of survival by hepatitis C virus (HCV) serostatus among HIV-infected subjects initiating their first antiretroviral therapy, showing deaths from natural causes only (excluding, for example, drug-overdose and traffic fatalities, suicides and homicides). The graph on the right includes data from only those subjects who were at least 95% adherent to their therapy regimen.
Table 2 summarizes the unadjusted and adjusted hazards of death from natural causes and HIV-related death. After controlling for sex, age at baseline, having a history of injection drug use, adherence to ART, having an AIDS diagnosis at baseline, time-updated CD4 count and log HIV viral load, HCV serostatus remained strongly predictive of death in this population (adjusted HR 2.20, 95% CI 1.50– 3.21, p < 0.001). Adjusting for the same factors, HCV coinfection remained independently predictive of an HIV-related death (adjusted HR 1.75, 95% CI 1.13–2.72, p = 0.012).
Table 2
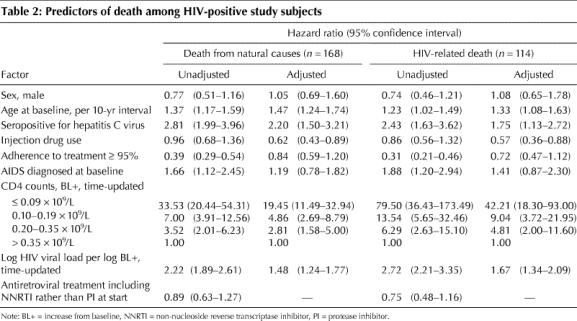
Injection drug use was examined for interaction with HCV seropositivity and with treatment adherence. Because no evidence was found of an interaction in either case, neither was included in the final models.
Interpretation
In this previously ART-naive HIV-infected population receiving highly active antiretroviral therapy, our findings strongly supported the hypothesis that HCV seropositivity is an independent predictor of death, particularly HIV-related death. Our data are consistent with findings from other studies4,5,20 that have shown that HCV coinfection has an adverse impact on HIV disease progression and HIV-related mortality.
HCV infection could affect HIV-related survival and mortality in several ways. Liver injury and increased ART toxicity may preclude patients coinfected with HIV and HCV from tolerating antiretroviral therapy.21,22 Because the progression of HCV disease is exacerbated in the setting of HIV infection,16 cause of death may be noted as being only HIV-related.
This analysis had 4 key strengths. Because the data were drawn from an HIV population–based cohort, our findings are more generalizeable than those from other studies. By adjusting for adherence to antiretroviral medications and a history of injection drug use, we were able to at least partially account for 2 potentially important confounding factors. Because the study was restricted to people who initiated ART since 1996, this analysis was not subject to the kind of survivorship bias inherent in studies that analyzed the survival of coinfected individuals before and after 1996.9,20 And by removing data for deaths without a natural cause and (in the HIV subanalysis) non–HIV-related events, we have conservatively biased our estimates.
This analysis also had 5 potential limitations. First, the HCV data were based on serologic testing alone and not confirmed with polymerase chain reaction testing. Roughly 5% of seronegative people will have detectable HCV RNA,23 and 5%–10% of HIV-infected patients who are HCV seropositive will nevertheless have undetectable HCV RNA;24 this suggests that the potential for misclassification bias is minimal. Second, our measure of adherence was restricted to the first year of therapy; this was done explicitly to avoid the possible reverse- causation that may result from patients who become less adherent to their ART because they are too sick to take the medications. Furthermore, this measure may be limited because it is a proxy for actually swallowing pills, but it has been strongly validated with both surrogate and clinical outcomes.17,19 Third, although we have been able to adjust for the confounding effect of any history of injection drug use, this variable is not a marker of current use, and residual confounding may therefore be present. Our finding that any injection drug use in adjusted analyses was associated with about a 40% reduced risk of mortality may be related to residual confounding. Fourth, we were unable to account for the duration of HIV infection. Finally, because our centre has previously shown that 30% of people in British Columbia dying of HIV-related causes have never received antiretroviral therapy,25 and because the sociodemographic profile of these patients is consistent with people expected to be infected with both HIV and HCV in British Columbia, it is reasonable to suggest that the findings of our present study may be underestimates.
In summary, in this population-based study of HIV-infected people receiving their initial antiretroviral therapy, these data have suggested that HCV seropositivity is independently predictive of increased mortality, particularly HIV-related death, even after controlling for lack of adherence to antiretroviral drug therapy and history of injection drug use. Further work is needed to fully characterize the mechanisms responsible for the increased mortality observed among patients infected with both the human immunodeficiency and hepatitis C viruses.
Supplementary Material
Acknowledgments
We thank the staff of the John Ruedy Immunodeficiency Clinic at St. Paul's Hospital in Vancouver, and the staff of the HIV/AIDS Drug Treatment Program at the British Columbia Centre for Excellence in HIV/AIDS. We also wish to acknowledge the patients whose lives and deaths formed the basis for this analysis.
Footnotes
This article has been peer reviewed.
Contributors: Paula Braitstein conceived the idea and methods for the analysis and was primarily responsible for the analysis and writing of the manuscript. Benita Yip was the data manager and senior statistician on the project. Robert Hogg was responsible for the data set. Valentina Montessori, David Moore and Julio Montaner were consultant physicians throughout the development of the manuscript.
All authors approved revisions of the manuscript before its resubmission and approved the final submitted version.
This manuscript was made possible through Doctoral Fellowships to Paula Braitstein and a Senior Scholar Award to Robert Hogg by the Michael Smith Foundation for Health Research and by a Doctoral Fellowship from the Canadian Institutes for Health Research to Paula Braitstein.
Competing interests: Robert Hogg had travel assistance to attend 2004 and 2005 European cohort conferences sponsored by GlaxoSmithKline, as did David Moore to attend a 2005 workshop on HIV databases in Hungary. Julio Montaner has received grants from, served as an ad hoc advisor to or spoken at various events sponsored by Avexa Ltd., Abbott Laboratories, Agouron Pharmaceuticals Inc., Boehringer Ingelheim Pharmaceuticals Inc., Borean Pharma AS, Bristol–Myers Squibb, DuPont Pharma, Gilead Sciences, GlaxoSmithKline, Hoffmann–La Roche, Immune Response Corporation, Janssen– Ortho Inc., Kucera Pharmaceutical Company, Merck Frosst Laboratories, Pfizer Canada Inc., Pharmacia & Uphohn, Sanofi Pasteur, Shire Biochem Inc., Tibotec Pharmaceuticals Ltd. and Trimeris Inc.
Correspondence to: Dr. Paula Braitstein, Department of Social and Preventive Medicine, University of Berne, Finkenhubelweg 11, CH-3012 Berne, Switzerland; fax 011-41-31-631-3520; pbraitstein@ispm.unibe.ch
References
- 1.Rockstroh JK, Spengler U. HIV and hepatitis C virus co-infection [review]. Lancet Infect Dis 2004;4:437-44. [DOI] [PubMed]
- 2.Cacoub P, Geffray L, Rosenthal E, Perronne C, Veyssier P, Raguin G; Joint Study Group on Hepatitis C Virus of the French National Society of Internal Medicine and the French Society of Infectious Diseases (GERMVIC Study Group). Mortality among human immunodeficiency virus–infected patients with cirrhosis or hepatocellular carcinoma due to hepatitis C virus in French departments of internal medicine/infectious diseases, in 1995 and 1997. Clin Infect Dis 2001;32:1207-14. [DOI] [PubMed]
- 3.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 2001;32:492-7. [DOI] [PubMed]
- 4.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study [published erratum appears in Lancet 2001;357(9267):1536]. Lancet 2000;356(9244):1800-5. [DOI] [PubMed]
- 5.Daar ES, Lynn H, Donfield S, Gomperts E, O'Brien SJ, Hilgartner MW, Hoots WK, et al. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis 2001;183:589-95. [DOI] [PubMed]
- 6.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA 2002;288:199-206. [DOI] [PubMed]
- 7.Cooper CL. Natural history of HIV and HCV coinfection [review]. J Int Assoc Physicians AIDS Care (Chic Ill) 2003;2:147-51. [PubMed]
- 8.Law WP, Duncombe CJ, Mahanontharit A, Boyd MA, Ruxrungtham K, Lange JM, et al. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS 2004;18:1169-77. [DOI] [PubMed]
- 9.Qurishi N, Kreuzberg C, Luchters G, Effenberger W, Kupfer B, Sauerbruch T, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 2003;362(9397):1708-13. [DOI] [PubMed]
- 10.Strathdee S, Patrick DM, Currie SL, Cornelisse PG, Rekart ML, Montaner JS, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS 1997;11(8):F59-65. [DOI] [PubMed]
- 11.Patrick DM, Tyndall MW, Cornelisse PG, Li K, Sherlock CH, Rekart ML, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ 2001;165(7):889-95. [PMC free article] [PubMed]
- 12.Hogg RS, Yip B, Kully C, Craib KJ, O'Shaughnessy MV, Schechter MT, et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ 1999;160(5):659-65. [PMC free article] [PubMed]
- 13.Yeni PG, Hammer SM, Carpenter CC, Cooper DA, Fischl MA, Gatell JM, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society — USA Panel [published erratum appears in JAMA 2003;11:32]. JAMA 2002;288:222-35. [DOI] [PubMed]
- 14.World Health Organization. International classification of diseases. 9th (1975) rev. Geneva: the Organization; 1977.
- 15.World Health Organization. International statistical classification of diseases and related health problems. 10th rev. Geneva: the Organization; 1992.
- 16.Soriano V, Puoti M, Sulkowski M, Mauss S, Cacoub P, Cargnel A, et al. Care of patients with hepatitis C and HIV co-infection [review]. AIDS 2004;18:1-12. [DOI] [PubMed]
- 17.Low-Beer S, Yip B, O'Shaughnessy MV, Hogg RS, Montaner JS. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr 2000;23: 360-1. [DOI] [PubMed]
- 18.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Is there a baseline CD4 cell count that precludes a survival response to modern antiretroviral therapy? AIDS 2003;17:711-20. [DOI] [PubMed]
- 19.Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O'Shaughnessy MV, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS 2002;16:1051-8. [DOI] [PubMed]
- 20.Klein M, Lalonde R., Suissa S. The impact of hepatitis C virus coinfection on HIV progression before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2003;33:365-72. [DOI] [PubMed]
- 21.Melvin D, Lee JK, Belsey E, Arnold J, Murphy RL. The impact of co-infection with hepatitis C virus and HIV on the tolerability of antiretroviral therapy. AIDS 2000;14:463-5. [DOI] [PubMed]
- 22.Ripamonti D, Arici C, Pezzotti P, Maggiolo F, Ravasio L, Suter F. Hepatitis C infection increases the risk of the modification of first highly active antiretroviral therapy in HIV-infected patients. AIDS 2004;18:334-7. [DOI] [PubMed]
- 23.George SL, Gebhardt J, Klinzman D, Foster MB, Patrick KD, Schmidt WN, et al. Hepatitis C virus viremia in HIV-infected individuals with negative HCV antibody tests. J Acquir Immune Defic Syndr 2002;31:154-62. [DOI] [PubMed]
- 24.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected patient [review]. Clin Liver Dis 2003;7:179-94. [DOI] [PubMed]
- 25.Wood E, Montaner JS, Tyndall MW, Schechter MT, O'Shaughnessy MV. Hogg RS. Prevalence and correlates of untreated human immunodeficiency virus type 1 infection among persons who have died in the era of modern antiretroviral therapy. J Infect Dis 2003;188:1164-70. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


