Abstract
Abstract
Objective
This study aimed to assess the effect of home-based exercise interventions on walking performance in patients with peripheral artery disease (PAD) and intermittent claudication (IC).
Design
Systematic review and meta-analysis.
Data sources
We searched the Medline, Web of Science, Embase, Scopus and Cochrane Library databases to identify randomised controlled trials of patients with PAD and IC published in English up to August 2024.
Eligibility criteria
Randomised controlled trials of patients who participated in home-based exercise interventions and were assessed for walking performance were eligible for inclusion. Studies without available data were excluded.
Data extraction and synthesis
We analysed the pooled effect size on walking performance based on the standardised mean differences between groups. A leave-one-out sensitivity analysis was performed to ensure the robustness of the findings.
Results
A total of eight studies were included in the meta-analysis. The duration of interventions in the included studies ranged from 6 to 52 weeks. In the pooled analysis, compared with control groups, the home-based exercise intervention groups showed improved pain-free walking distance (standardised mean difference 0.67, 95% CI 0.20 to 1.15), and maximal walking distance (0.47, 0.05 to 0.89). The overall heterogeneity score of pain-free walking distance was I2=83% (p<0.001), and for maximal walking distance, I2=78% (p<0.001).
Conclusions
Home-based exercise interventions for patients with PAD and IC were beneficial in improving pain-free walking distance and maximal walking distance. Future studies should consider multiple factors that may affect the effectiveness of home training and intervention compliance.
Trial registration number
PROSPERO, CRD42024499020.
Keywords: Clinical Trial, SPORTS MEDICINE, Cardiovascular Disease, Meta-Analysis
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This systematic review and meta-analysis was based on a thorough search across databases for randomised clinical trials (RCTs) of home-based exercise for patients with peripheral artery disease and intermittent claudication symptoms.
The overall quality of the studies was moderate, with half showing a moderate risk of bias and half showing a low risk.
Few studies met inclusion criteria, limiting the analysis’ statistical power and generalisability.
There was considerable heterogeneity between studies, complicating the derivation of precise exercise prescriptions.
Introduction
Since Alan Hirsch described peripheral artery disease (PAD) as the ‘last major pandemic of cardiovascular disease’, it has, as of 2019, affected an estimated 236.6 million individuals globally, with 172.5 million residing in low-income and middle-income countries and 64.1 million in high-income countries.1 PAD encompasses a broad spectrum of conditions, from asymptomatic stages to intermittent claudication (IC), severe limb ischaemia, and ultimately, limb loss.2 3 IC, the most common symptom, presents as spasmodic leg pain during walking, alleviated with brief rest.4 This symptom significantly limits the maximum distance patients can walk without pain, restricts physical activity, and markedly diminishes health-related quality of life.5 Moreover, IC is intricately linked with an increased risk of cardiovascular diseases and mortality due to the systemic nature of atherosclerosis.6 The 5-year all-cause mortality rate for individuals with IC is estimated at 10–15%, potentially rising to 25% within 1 year if IC progresses to critical limb ischaemia.7
Exercise capacity is a robust predictor of mortality among patients with PAD,8 and physical activity is known to offer a protective effect against mortality for those with claudication resulting from PAD.9 Supervised exercise programmes are considered the primary treatment for walking impairments in patients with PAD.10 Yet, the logistical demands of participating in these programmes are significant, leading to low enrolment among those with PAD.11 Home-based exercise, which involves self-directed exercises under medical advice either at home or in community settings, presents a potentially less burdensome alternative. However, the efficacy of these home-based walking exercises for PAD remains unclear.12 13
Previous systematic reviews have primarily focused on supervised exercise programmes,14 15 home-based exercise programmes,16 and the cumulative effects of various interventions on physical activity and fitness in patients with PAD or IC.17 However, there has been limited attention specifically on patients with PAD and IC. It is important to note that most patients with PAD do not exhibit typical IC symptoms. To address this gap, we conducted a systematic review and meta-analysis to evaluate the impact of home-based exercise on walking performance specifically in patients with PAD and classic IC symptoms. Primary outcomes included the maximal walking distance (MWD) and pain-free walking distance (PFWD) for the walking test. This investigation aims to provide a clearer understanding of the potential benefits of home-based exercise interventions, thereby offering valuable insights into the management of PAD and IC.
Methods
Data sources and search strategies
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline18 and the Cochrane Collaboration recommendations.19 The protocol (PROSPERO CRD42024499020) was registered and published on PROSPERO without amendments.
We searched five databases—Medline (via PubMed), Web of Science, Embase, Scopus, and the Cochrane Library—up to August 2024. In Medline, we initially searched using subject headings and keywords in the titles and abstracts. The initial keywords used in the Medline search were: peripheral arterial disease, arterial disease peripheral, disease peripheral arterial, peripheral arterial disease*, artery disease peripheral, disease peripheral artery, peripheral artery diseases, intermittent claudication, home, residential*, exercise*, physical activit*, activit* physical, training*, exercise*, 6 min* walk, 6 min walk, 6 min walk. A comprehensive search of all databases was then conducted using the identified subject headings and keywords. Finally, reference list searches of the retrieved articles were performed to locate additional references. The complete search strategy for the five databases is provided in online supplemental table 1.
Inclusion and exclusion criteria
The inclusion criteria for this review were meticulously defined following the Population, Intervention, Comparison, Outcome (PICO) framework to ensure clarity and precision. The population of interest comprised adult patients diagnosed with PAD and who were experiencing IC. The intervention focus was home-based exercise, characterised by physical activities performed within or near the participant’s home, including areas such as gardens and driveways.20 This definition was adopted to isolate the direct effects of exercise from those of other interventions. For comparison, the study imposed no restrictions on the control types utilised in the evaluated research. Outcomes of interest were primarily the walking ability measured using the 6-min walk test (6-MWT). This review exclusively included randomised controlled trials (RCTs) that were published as full-text articles. Exclusion criteria encompassed protocols, studies without available full texts, abstracts presented in conference proceedings, and letters to the editor, ensuring that only the most rigorous and comprehensive data were analysed. The language of publication was restricted to English to facilitate the review process, although no limitations were placed on the publication year, allowing for a broad examination of relevant literature. This approach aimed to synthesise high-quality evidence on the effectiveness of home-based exercise interventions for individuals with PAD and IC, providing a foundation for informed clinical practice and future research directions.
Study selection
Based on the search strategy, studies were retrieved from the five databases, and duplicates were removed. Two authors (ZX and JC) independently screened the titles and abstracts of the remaining studies to determine their eligibility for the study based on the inclusion and exclusion criteria. The full texts of the selected studies were subsequently assessed and the final studies to be included in the analysis were selected. Thereafter, we manually searched the reference lists of all included studies for additional relevant studies. In instances where there were disagreements regarding decisions, a third author (XZ) participated in discussions until a consensus was reached.
Risk of bias and quality assessment
Two authors (ZX and JC) independently assessed the risk of bias following the Cochrane risk-of-bias tool (RoB2),21 which focuses on seven domains: random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other sources of bias (each scored as high risk, low risk, or unclear). In the case of disagreement, a third person (X-QZ) joined discussions to resolve any disagreements.
Data extraction
Two authors (ZX and JC) independently extracted the data elements from the studies included in the final analysis. The datasheet contained fields for the first author, year of publication, country, participants, sample size, age of participants, study duration, intervention, comparator and outcomes. If some elements of the desired data were not reported in a study, we contacted the corresponding author of the study to obtain these data.
Where reported, the mean and standard deviation (pre-intervention and post-intervention or control) were extracted. The mean change in each outcome (PFWD and MWD) for each group was calculated by subtracting the immediate post-intervention mean from the pre-intervention mean, and the SD of the change was calculated assuming a conservative correlation coefficient of 0.5.
Data synthesis
Pooled effect sizes were estimated using random-effect models, which used the mean and SD of the change in each outcome from baseline. Because of the use of different scales to measure the same construct across the studies, the summary effects were expressed as standardised mean differences (SMDs) between groups, with corresponding 95% confidence intervals (CIs) and p values.22 An SMD of less than 0.2 was considered a small effect size, 0.5 a medium effect size and 0.8 a large effect size.23 The statistical heterogeneity of the included studies was quantified by the I2 statistic, with 25%, 50% or 75% reflecting low, moderate or high heterogeneity, respectively.22
When possible, data from the included studies were used for the following comparisons: home-based exercise versus no-treatment control, and home-based exercise versus supervised exercise groups. All analyses were conducted using RevMan 5.4, with statistical significance set at p<0.05.
Quality of evidence
For each outcome, two reviewers (ZX and JC) used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to assess the certainty of the evidence, as described elsewhere.24 GRADE ratings for each outcome started at ‘high’ due to the RCT design. Downgrading was determined by the factors of risk of bias, inconsistency, indirectness, and imprecision.25
Sensitivity analysis
We conducted a sensitivity analysis to evaluate the robustness of the results obtained from the combined analysis of the eight studies. The main approach involves systematically excluding one study at a time and re-analysing the data with the remaining seven studies (the leave-one-out approach). This process is repeated for each of the eight studies in turn.
Patient and public involvement
None.
Results
Study selection
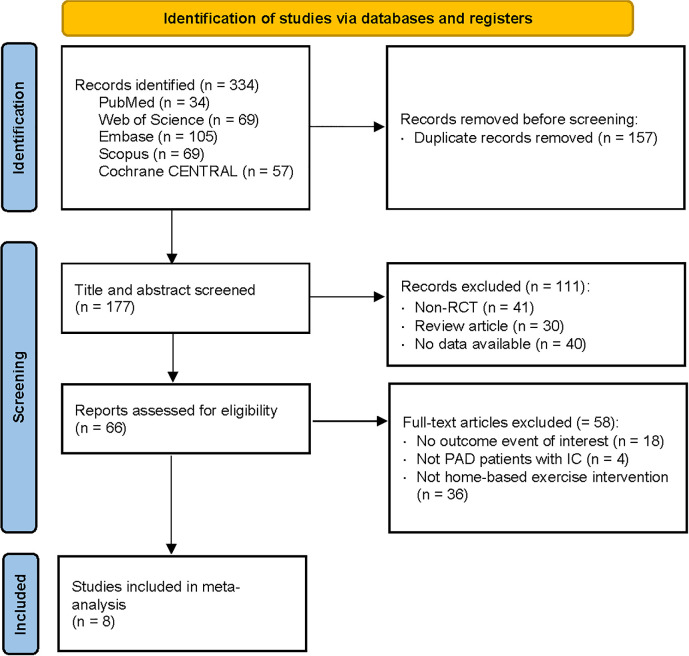
Figure 1 shows our search process and the results obtained through our search strategy in a PRISMA flowchart.
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart of the study selection process.46 PAD: peripheral artery disease; RCT: randomised controlled trial.
We identified a total of 334 articles from the search of five databases. After we eliminated duplicates, 177 articles remained. Of these remaining articles, a further 111 were excluded based on title and abstract screening. The most common reason for exclusion during the eligibility phase was that studies either did not assess clinical outcomes (eg, maximal walking time not measured during the 6-MWT) or involved patients with PAD but without IC symptoms. Finally, eight studies were included in the meta-analysis. This systematic review includes studies from the inception of each database through August 2024.
Study characteristics
The characteristics of the eight studies included are presented in table 1. All of the included studies were RCTs. Of the studies, six were undertaken in Europe,26,31 one in North America32 and one in Africa.33 All participants were diagnosed with PAD and IC. The number of participants varied from 22 to 148, and the mean age of the participants was over 57 years. Of the studies, three compared home-based exercise with supervised exercise groups,30 32 33 and the remaining seven studies compared the effects of home-based exercise with the usual care group, except for one study.33 Regarding the intervention duration of the included studies, three studies were performed for 12 weeks,27 28 32 two studies were performed for 26 weeks,29 31 and there was one study each with intervention durations of 6,33 1626 and 52 weeks.30
Table 1. Characteristic of the included studies (n=8).
| Study | Country | Diagnostic criteria | Sample size (n) | Age of participants (years), mean (SD) | Adherence rate | Study duration (weeks) |
| Parr et al, 200933 | South Africa | Duplex flow Doppler | UBST group: 9; CER group: 8; CONT group: 8 | UBST group: 66 (13);CER group: 57 (14);CONT group: 62 (10) | Not reported | 6 |
| Jakubsevičiene et al, 201429 | Lithuania | Stage II–III PAD as defined by Fontaine | Intervention group: 57;Control group: 60 | Intervention group: 67.4 (1.0);Control group: 66.5 (1.0) | Not reported | 26 |
| Gardner et al, 201432 | America | Ambulatory leg pain confirmed by treadmill exercise with an ankle-brachial pressure index (ABI)≤0.90 at rest or ≤0.73 after exercise | Supervised exercise intervention: 60;Home-based exercise intervention: 60;Attention control: 60 | Supervised exercise intervention: 65 (11);Home-based exercise intervention: 67 (10);Attention control: 65 (9) | 81%81% | 12 |
| Galea Holmes et al, 201926 | United Kingdom | Diagnosed by a vascular clinician and confirmed by the San Diego Claudication Questionnaire | Treatment group: 12;Attention control group: 10 | Treatment group 66.3 (8.8);Attention control group 67.1 (11.2) | 67%90% | 16 |
| Bearne et al, 202227 | United Kingdom | Determined by the consulting clinician based on ABI≤0.90 and self-reported claudication identified using the San Diego Claudication Questionnaire | Intervention group: 74;Usual care group: 74 | Intervention group 67.6 (8.7);Usual care group 68.2 (9.0) | 79%Not reported | 12 |
| Sandberg et al, 202330 | Sweden | Mild-to-severe IC (Rutherford categories 1–3) for >6 months, confirmed with an ABI<0.9, and/or a post-exercise ABI drop ≥30% | SEP group: 48;HSEP group: 50;WA group: 47 | 72.1 (7.4) | 74%95%Not reported | 52 |
| Manfredini et al, 202431 | Italy | PAD patients at Leriche-Fontaine stage IIa or IIb | TiTo-SHB: 34;C-WA: 34 | TiTo-SHB: 71 (7);C-WA: 73 (7) | 94%54% | 26 |
| Waddell et al, 202428 | United Kingdom | Vascular clinics with confirmed symptomatic PAD (ABI<0.90) | Home-based exercise: 16;Non-exercise: 14 | Home-based exercise: 68.3 (9.6);Non-exercise: 68.1 (8.5) | 53.5% | 12 |
CERconventional exercise rehabilitation programmeCONTwalk at homeHSEPhome-based structured exercise programmeSEPhospital-based supervised exercise programmeTiTo-SHB’Test in-Train out' structured home-based exercise programmeUBSTupper body strength training programmeWAwalk advice
Risk of bias and quality assessment
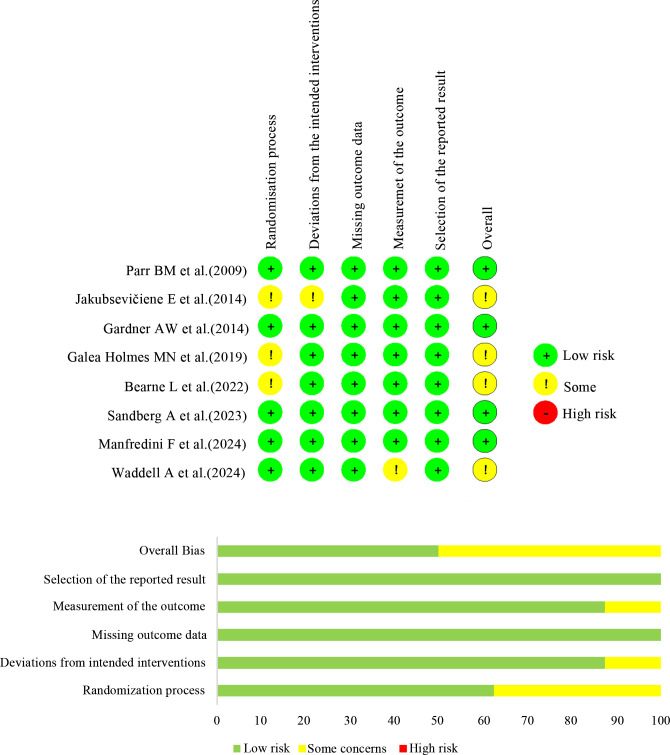
Risk of bias judgements are presented in figure 2. For lack of information on concealment, three of the eight studies were assessed as some concerns about the randomisation process.26 27 29 One study was evaluated as some concerns because it did not report whether the trial context deviated from the intended intervention.29 One study was rated as some concerns due to lack of information about the evaluators’ knowledge about the intervention.28 And missing outcome data, and selection of the reported result were generally assessed to have a low risk. Additionally, publication bias was assessed using funnel plot points, which appeared asymmetrical, as depicted in online supplemental figure 1. However, Egger’s test for intercept did not reveal any publication bias (all p values>0.05).
Figure 2. Risk of bias: individual studies.
The quality of evidence was assessed using the GRADE system. The results indicated that, compared with the control, the evidence quality for PFWD and MWD was low, and the quality for PFWD and MWD was rated as moderate and high when compared with supervised exercise, respectively (online supplemental table 2).
Characteristics of study outcomes
All studies included comparators, and they are presented with the intervention methods and outcomes in table 2.
Table 2. Characteristics of the study intervention and outcomes.
| Study | Intervention | Follow-up assessment | Outcomes |
| Parr et al, 200933 | Home-based exercise: advised to ‘walk as much as possible at home’Supervised exercise: conventional exercise and upper body strength training groups attended structured exercise 3 times a week for a 45 min period for 6 weeks | Baseline and 6 weeks | PFWD: increased significantly compared with the CONT group (p=0.03)MWD: no significant difference in mean change |
| Jakubsevičiene et al, 201429 | Procedures consisted of the following: (1) a 5–10 min warm-up; (2) lower limb exercising; (3) a 5–10 min cool-down | Baseline, after 1 month and 6 months | PFWD and 6-MWT improved significantly in the intervention group compared with the control group |
| Gardner et al, 201432 | Home-based exercise: 3 months of intermittent walking to mild-to-moderate claudication pain 3 days per week at a self-selected paceSupervised exercise: 3 months of intermittent treadmill walking to mild-to-moderate claudication pain 3 days per week at a speed of ≈2 mph | Baseline and 12 weeks | 6-MWT total distance: intervention groups increased significantly compared with baseline (p<0.05), and the changes in the home-based exercise group were higher than those in the other two groups |
| Galea Holmes et al, 201926 | MOSAIC treatment, including two 60 min home-based sessions and two 20 min booster telephone calls incorporating behaviour-change techniques | Baseline and 16-week follow-up | MWD: decreased in treatment group |
| Bearne et al, 202227 | Two 60 min in-person sessions and two 20 min telephone sessions delivered by physical therapists | Baseline and 3 months | PFWD: increased significantly compared with the usual care group (p=0.02)MWD: increased significantly compared with the usual care group (p=0.009) |
| Sandberg et al, 202330 | Home-based exercise: aerobic walking and resistance exercises to be perform three times weekly at homeSupervised exercise; same exercise description as HSEP but under supervision from physiotherapist | Baseline, 3, 6 and 12 months | PFWD: no significant difference in mean changeMWD: no significant difference in mean change |
| Manfredini et al, 202431 | The programme included two daily 8 min sessions of pain-free interval walking at progressive low to moderate speed maintained with a metronome | Baseline and 6 months | PFWD and MWD in TiTo-SHB group significantly improved compared with the C-WA group (p<0.001) |
| Waddell et al, 202428 | The HSEP group was given a Fitbit to use during a 12 week exercise programme comprising personalised step goals and a resistance-based circuit to be undertaken at home twice weekly | Baseline and 12 weeks | There were no significant differences in PFWD and MWD between groups at 12 weeks, but minimally clinically important difference was seen in PFWD in both groups |
CONTwalk at homeHSEPhome-based structured exercise programmeMOSAICMotivating Structured walking Activity in Intermittent ClaudicationMWDmaximal walking distance6-MWT6-min walk testPFWDpain-free walking distance
In all studies, walking performance was measured through 6-MWT at baseline and at the end of the study or at certain time points during the study. Six trials used PFWD to present the results of 6-MWT. Two trials showed no significant change,28 30 and the remaining four trials all showed significant improvements.27 29 31 33 All studies in 6-MWT results opted to use the MWD indicator, with three studies showing no significant change,28 30 33 four exhibiting a noticeable increase,27 29 31 32 and one demonstrating a decrease.26
Meta-analysis findings: effects of home-based exercise interventions
Home-based exercise versus control
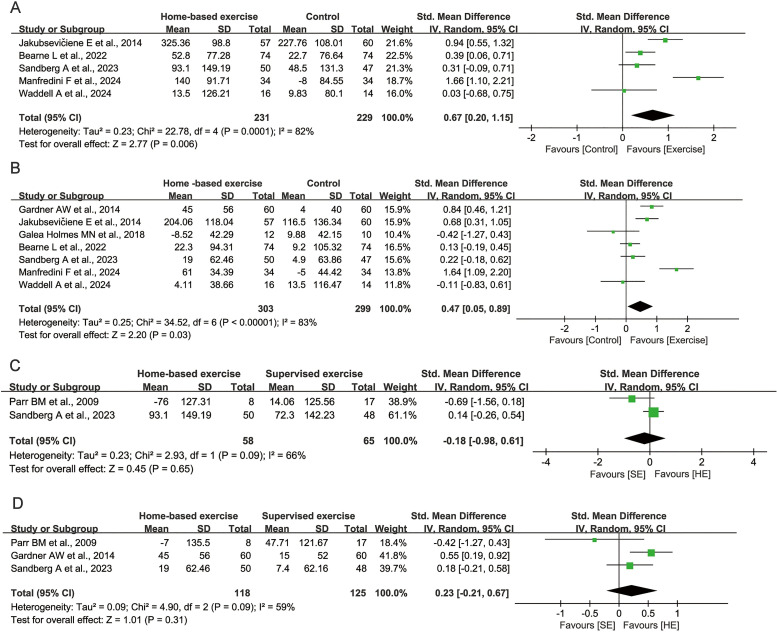
All eight studies included in the meta-analysis reported walking performance using the 6-MWT. Compared with the control group, the home-based exercise group showed an overall improvement in PFWD (SMD=0.67, 95% CI 0.20 to 1.15, p=0.006; figure 3A), and MWD (SMD=0.47, 95% CI 0.05 to 0.89, p=0.03; figure 3B), as assessed in seven studies. The pooled analysis revealed significant heterogeneity in PFWD (I2=82%, p<0.05), and MWD (I2=83%, p<0.05) results. Even though a leave-one-out sensitivity analysis was performed, we observed that no primary study was a significant source of heterogeneity for PFWD (online supplemental table 3) and MWD (online supplemental table 4).
Figure 3. Forest plot of walking performance (up to 52-week point). (a) PFWD of home-based exercise compared with control group; (b) MWD of home-based exercise compared with control group; (c) PFWD of home-based exercise (HE) compared with supervised exercise (SE); (d) MWD of home-based exercise compared with supervised exercise. MWD: maximal walking distance; PFWD: pain-free walking distance.
Home-based exercise versus supervised exercise
Compared with supervised exercise groups, home-based exercise groups showed a decrease in walking performance of patients with PAD and IC, mainly manifested in MWD (SMD=0.23, 95% CI −0.21 to 0.67, p=0.31; figure 3D), but PFWD showed no change (SMD=−0.18, 95% CI −0.98 to 0.61, p=0.65; figure 3C). Both exhibit moderate heterogeneity. For MWD, in leave-one-out sensitivity analysis, omitting Parr et al, there was a decrease from 59% to 44% in I2 (online supplemental table 5). Since PFWD has been incorporated into relatively few studies, it is not possible to conduct a sensitivity analysis (online supplemental table 6).
Discussion
This study presents a comprehensive meta-analysis evaluating the efficacy of home-based exercise as a treatment method for patients with PAD experiencing IC. Although supervised exercise is considered the first-line therapy for patients with PAD to improve walking ability, travelling to a centre for supervised exercise regularly poses huge challenges with regard to transportation, cost and resources availability.34 35 Home-based exercise emerges as a more accessible and cost-effective alternative to supervised exercise, reducing both burden and expense.36 Parr and colleagues34 reported supervised exercise therapy provided an important benefit for MWD and PFWD compared with home-based exercise therapy. The upper-body strength training and dynamic exercise programme demonstrated positive effects on 6-MWT in patients with PAD and IC. In contrast, home-based exercise primarily involved walking at home without the structured programme found in other studies. This difference in exercise approach may contribute to the high heterogeneity in our results. Gardner and colleagues33 reported NEXT Step home exercise with favourable results for 6MWD. Sandberg and colleagues,31 who prescribed three sessions per week of aerobic walking and resistance exercise for the home-based structured exercise programme and unsupervised walking advice for the control group, reported non-inferior effects from supervised exercise for PFWD and MWD. Reviewing the two studies in detail, several study and exercise characteristics varied, but nevertheless both trials applied predominately unsupervised exercise protocols. These findings, along with walking performance data, supported the hypothesis that supervision may not be essential for achieving improvements in walking performance. In summary, we observed similar effects of predominately unsupervised versus supervised exercise protocols.
Apart from effectiveness, compliance with home-based exercise programmes is identified as a key determinant of their success. Despite limited reporting, with six studies detailing compliance rates, findings suggest that adherence levels in these programmes are promising, with rates surpassing 53%. This is slightly lower than compliance rates observed in elderly exercise programmes (65–86%),37 PAD patient exercise groups (78%),38 and PAD interventions utilising mobile health technologies (80%).17 In a recent meta-analysis on supervised exercise effects that confirmed the present results for home-based exercise, Pymer and colleagues39 emphasise several reasons for the superiority of unsupervised (home-based) exercise programmes that are predominately related to monitoring, exercise description, education, goal setting and action planning. Particularly, the structure of unsupervised exercise protocols has to be emphasised and indicated that walking performance improvement cannot be fully attributed to the degree of supervision but in part to exercise characteristics closely related to unsupervised walking. The challenge of achieving high compliance in unsupervised settings highlights the potential benefit of integrating supervisory elements to bolster adherence. Future investigations should explore diverse supervisory methods to enhance compliance and establish comprehensive metrics for measuring adherence in PAD and IC contexts. Another reason for the high heterogeneity when comparing home-based exercise groups with control groups might be the effect of the severity of PAD and IC. Different criteria for the diagnosis of PAD led to differences in disease severity among individuals. Despite high degree of heterogeneity between trials, our sensitivity analyses consistently demonstrated the robustness of the positive effects of home-based exercise.
Labs et al40 only discussed the type of exercise for patients with PAD without providing standardised data to support their findings. In contrast, Fokkenrood et al41 conducted a comparative analysis of the effects of supervised versus home-based exercise on the maximum walking distance or time for patients with IC, and updated their research in 201842 to emphasise measurements of maximum walking distance and peak walking distance using a treadmill. Our study specifically targets patients with classic symptoms of IC associated with PAD. Similarly, Li et al16 and Thangada et al15 also focused on populations with IC. Additionally, van den Houten et al43 analysed the impacts of supervised exercise therapy, home-based exercise, and revascularisation on physical activity, quantified by daily step counts. In contrast, our research specifically examines the impact on walking ability. Therefore, compared with previously published meta-analyses, our study is distinctly focused on patients with PAD exhibiting claudication symptoms, utilising 6-MWT to assess walking capacity, enhancing the specificity of the research.
Our meta-analysis, incorporating eight studies, reveals that home exercise interventions exhibit variable impacts on the 6-MWT outcomes among patients with PADd and IC. The variability in performance improvements, particularly in PFWD, appears to correlate with the intervention’s duration (online supplemental figure 2). Notably, mere walking advice without structured supervision did not significantly enhance MWD. This suggests a nuanced interaction between intervention strategies and their effectiveness, underscoring the need for more structured and supervised home training programmes to achieve meaningful improvements. Apart from the direct negative effect of less sophisticated exercise programmes in meta-analysis results, another aspect has to be considered. In contrast to methodological quality,44 there is still no recognised score available to determine the quality of the unsupervised exercise intervention and thus enable appropriate weighting of the studies. Both features definitely represent a major limitation of meta-analysis in the area of exercise and hinder the achievement of higher exercise effects.45
The strength of our study is that it summarises results of all published studies to date and provides evidence for the advantages of home-based exercise for patients with PAD and IC. We used a standardised methodology for conducting this systematic review and meta-analysis, registered with PROSPERO, a comprehensive search strategy, with appropriate quality assessment of studies included in the systematic review with RoB2 and standardised reporting of systematic reviews with the PRISMA checklist. In addition, we would like to briefly address the limitations of the present work. First, the intervention protocols of home exercise between studies varied, contributing to high heterogeneity, which complicates the derivation of precise exercise prescriptions for effective intervention. Second, in contrast to supervised exercise, we included a trial with ‘walk at home’ as the home-based exercise group; this may weaken the observed effect of home-based exercise, which is structured exercise in other trials. The complexity of these interventions underscores the need for further research to delineate the specific effects of various home training modalities, including those based on mobile technology and behavioural change theories. Lastly, because of the small sample size and low quality of evidence, the findings from our work needs to be considered when applying the results. The scarcity of included studies reflects the broader lack of research in this area, indicating a critical need for expanded investigation into the impact of home-based training on patients with PAD and IC. In summary, the present systematic review and meta-analysis provided evidence for positive effects of home-based exercise on patients with PAD and IC. Considering the importance of supervision of the exercise programme, regular feedback, such as step activity monitors, may improve the adherence of patients with PAD to exercise programmes, thereby increasing the effectiveness of home-based exercise.
Conclusions
This meta-analysis provides preliminary evidence that home-based exercise interventions may achieve modest improvements in 6-MWT outcomes, including PFWD and MWD, for patients with PAD and IC. This underscores the potential of home-based exercise programmes as a viable treatment modality. However, the findings also underscore the need for further research into intervention design intricacies, compliance factors, and their combined impact on the efficacy of home-based training. Future studies should aim to address these gaps and provide clearer guidance for optimising exercise interventions for this patient population.
supplementary material
Footnotes
Funding: This research was funded by Shanxi Office of Philosophy and Social Science (2022YY037).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-086013).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data availability statement
Data are available upon reasonable request.
References
- 1.Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–30. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res. 2015;116:1540–50. doi: 10.1161/CIRCRESAHA.114.303517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costantini V, Lenti M. Treatment of acute occlusion of peripheral arteries. Thromb Res. 2002;106:V285–94. doi: 10.1016/s0049-3848(02)00104-4. [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45 Suppl S:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Li YH, Yeh HI, Hwang JJ. Antithrombotic Treatment for Symptomatic Peripheral Artery Disease. Acta Cardiol Sin. 2019;35:557–62. doi: 10.6515/ACS.201911_35(6).20190907A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Criqui MH, Coughlin SS, Fronek A. Noninvasively diagnosed peripheral arterial disease as a predictor of mortality: results from a prospective study. Circulation. 1985;72:768–73. doi: 10.1161/01.cir.72.4.768. [DOI] [PubMed] [Google Scholar]
- 7.Krishna SM, Moxon JV, Golledge J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int J Mol Sci. 2015;16:11294–322. doi: 10.3390/ijms160511294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leeper NJ, Myers J, Zhou M, et al. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2013;57:728–33. doi: 10.1016/j.jvs.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner AW, Montgomery PS, Parker DE. Physical activity is a predictor of all-cause mortality in patients with intermittent claudication. J Vasc Surg. 2008;47:117–22. doi: 10.1016/j.jvs.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallahtafti F, Salamifar Z, Hassan M, et al. Joint Angle Variability Is Altered in Patients with Peripheral Artery Disease after Six Months of Exercise Intervention. Entropy (Basel) 2022;24:1422. doi: 10.3390/e24101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Liu X, Wang W, et al. Adherence, Efficacy, and Safety of Wearable Technology-Assisted Combined Home-Based Exercise in Chinese Patients With Ankylosing Spondylitis: Randomized Pilot Controlled Clinical Trial. J Med Internet Res. 2022;24:e29703. doi: 10.2196/29703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bearne L, Galea Holmes M, Bieles J, et al. Motivating Structured walking Activity in people with Intermittent Claudication (MOSAIC): protocol for a randomised controlled trial of a physiotherapist-led, behavioural change intervention versus usual care in adults with intermittent claudication. BMJ Open. 2019;9:e030002. doi: 10.1136/bmjopen-2019-030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairman CM, Owens OL, Kendall KL, et al. Study protocol: investigating the feasibility of a hybrid delivery of home-based cluster set resistance training for individuals previously treated for lung cancer. Pilot Feasibility Stud . 2022;8:102. doi: 10.1186/s40814-022-01065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanigaimani S, Jin H, Silva MT, et al. Network Meta-Analysis of Trials Testing If Home Exercise Programs Informed by Wearables Measuring Activity Improve Peripheral Artery Disease Related Walking Impairment. Sensors (Basel) 2022;22:8070. doi: 10.3390/s22208070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thangada ND, Zhang D, Tian L, et al. Home-Based Walking Exercise and Supervised Treadmill Exercise in Patients With Peripheral Artery Disease: An Individual Participant Data Meta-Analysis. JAMA Netw Open . 2023;6:e2334590. doi: 10.1001/jamanetworkopen.2023.34590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Li Z, Chang G, et al. Effect of structured home-based exercise on walking ability in patients with peripheral arterial disease: a meta-analysis. Ann Vasc Surg. 2015;29:597–606. doi: 10.1016/j.avsg.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Kim C, Kim E, et al. Effectiveness of Mobile Health-Based Exercise Interventions for Patients with Peripheral Artery Disease: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth. 2021;9:e24080. doi: 10.2196/24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023) 2023.
- 20.Denton F, Power S, Waddell A, et al. Is It Really Home-Based? A Commentary on the Necessity for Accurate Definitions across Exercise and Physical Activity Programmes. Int J Environ Res Public Health. 2021;18:9244. doi: 10.3390/ijerph18179244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Chandler J, Cumpston M, Li T. Cochrane Handbook for Systematic Reviews of Interventions n.d.
- 23.Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P T. 2008;33:700–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Galea Holmes MN, Weinman JA, Bearne LM. A randomized controlled feasibility trial of a home-based walking behavior-change intervention for people with intermittent claudication. J Vasc Nurs. 2019;37:135–43. doi: 10.1016/j.jvn.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Bearne LM, Volkmer B, Peacock J, et al. Effect of a Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking in Adults With Peripheral Artery Disease. JAMA . 2022;327:1344. doi: 10.1001/jama.2022.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waddell A, Denton F, Powell R, et al. Home-based Circuit Training and Community Walking for Intermittent Claudication. Ann Vasc Surg. 2024;105:38–47. doi: 10.1016/j.avsg.2024.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Jakubsevičienė E, Vasiliauskas D, Velička L, et al. Effectiveness of a new exercise program after lower limb arterial blood flow surgery in patients with peripheral arterial disease: a randomized clinical trial. Int J Environ Res Public Health. 2014;11:7961–76. doi: 10.3390/ijerph110807961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandberg A, Bäck M, Cider Å, et al. Effectiveness of supervised exercise, home-based exercise, or walk advice strategies on walking performance and muscle endurance in patients with intermittent claudication (SUNFIT trial): a randomized clinical trial. Eur J Cardiovasc Nurs. 2023;22:400–11. doi: 10.1093/eurjcn/zvac070. [DOI] [PubMed] [Google Scholar]
- 31.Manfredini F, Traina L, Ficarra V, et al. A “test in-train out” program versus a “go home and walk” intervention for home-based exercise therapy in patients with peripheral artery disease: A randomized controlled trial. Scand J Med Sci Sports. 2024;34:e14584. doi: 10.1111/sms.14584. [DOI] [PubMed] [Google Scholar]
- 32.Gardner AW, Parker DE, Montgomery PS, et al. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3:e001107. doi: 10.1161/JAHA.114.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parr BM, Noakes TD, Derman EW. Peripheral arterial disease and intermittent claudication: efficacy of short-term upper body strength training, dynamic exercise training, and advice to exercise at home. S Afr Med J. 2009;99:800–4. [PubMed] [Google Scholar]
- 34.Treat-Jacobson D, McDermott MM, Bronas UG, et al. Optimal Exercise Programs for Patients With Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e10–33. doi: 10.1161/CIR.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 35.Harwood AE, Pymer S, Ibeggazene S, et al. Provision of exercise services in patients with peripheral artery disease in the United Kingdom. Vascular. 2022;30:874–81. doi: 10.1177/17085381211035259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowntree RA, Hosseinzadeh H. Lung Cancer and Self-Management Interventions: A Systematic Review of Randomised Controlled Trials. Int J Environ Res Public Health. 2022;19:536. doi: 10.3390/ijerph19010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picorelli AMA, Pereira LSM, Pereira DS, et al. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J Physiother. 2014;60:151–6. doi: 10.1016/j.jphys.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Lin E, Nguyen CH, Thomas SG. Completion and adherence rates to exercise interventions in intermittent claudication: Traditional exercise versus alternative exercise - a systematic review. Eur J Prev Cardiol. 2019;26:1625–33. doi: 10.1177/2047487319846997. [DOI] [PubMed] [Google Scholar]
- 39.Pymer S, Ibeggazene S, Palmer J, et al. An updated systematic review and meta-analysis of home-based exercise programs for individuals with intermittent claudication. J Vasc Surg. 2021;74:2076–85. doi: 10.1016/j.jvs.2021.03.063. [DOI] [PubMed] [Google Scholar]
- 40.Labs KH, Degischer S, Aschwanden M, et al. The value of walking exercise in treatment of intermittent claudication. Praxis. 1994;90:2056–9. [PubMed] [Google Scholar]
- 41.Fokkenrood HJP, Bendermacher BLW, Lauret GJ, et al. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2013:CD005263. doi: 10.1002/14651858.CD005263.pub3. [DOI] [PubMed] [Google Scholar]
- 42.Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;4:CD005263. doi: 10.1002/14651858.CD005263.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Houten MML, Hageman D, Gommans LNM, et al. The Effect of Supervised Exercise, Home Based Exercise and Endovascular Revascularisation on Physical Activity in Patients With Intermittent Claudication: A Network Meta-analysis. Eur J Vasc Endovasc Surg. 2019;58:383–92. doi: 10.1016/j.ejvs.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 44.Smart NA, Waldron M, Ismail H, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13:9–18. doi: 10.1097/XEB.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 45.Kemmler W. Meta-Analysis and Exercise Related Sports Medicine: WWF VERLAGSGESELLSCHAFT GMBH AM EGGENKAMP 37-39, 48268 Greven, Germany. 2013. pp. 96–7. [DOI]
- 46.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]