Abstract
Agrobacterium tumefaciens infects plants and induces the formation of tumors called “crown galls” by integrating the transferred-DNA (T-DNA) region of the Ti-plasmid into the plant nuclear genome. Tumors are formed because the T-DNA encodes enzymes that modify the synthesis of two plant growth hormones, auxin and cytokinin (CK). Here, we show that a CK biosynthesis enzyme, Tmr, which is encoded by the Agrobacterium T-DNA region, is targeted to and functions in plastids of infected plant cells, despite having no typical plastid-targeting sequence. Evidence is provided that Tmr is an adenosine phosphate-isopentenyltransferase (IPT) that creates a new CK biosynthesis bypass by using 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (HMBDP) as a substrate. Unlike in the conventional CK biosynthesis pathway in plants, trans-zeatin-type CKs are produced directly without the requirement for P450 monooxygenase-mediated hydroxylation. Consistent with the plastid localization of Tmr, HMBDP is an intermediate in the methylerythritol phosphate pathway, a plastid-localized biosynthesis route for universal isoprenoid precursors. These results demonstrate that A. tumefaciens modifies CK biosynthesis by sending a key enzyme into plastids of the host plant to promote tumorigenesis.
Keywords: crown gall, isopentenyltransferase, methylerythritol phosphate pathway
Cytokinins (CKs) are a group of plant hormones essential for cell division and differentiation in plants (1). Most natural CKs, including isopentenyladenine (iP) and trans-zeatin (tZ) (Fig. 1), are derivatives of N6-prenylated adenine (1). The prenyl side chain of CKs can be derived from the methylerythritol phosphate (MEP) pathway or the mevalonate (MVA) pathway, both of which supply common C5 units for isoprenoid biosynthesis (2, 3). The MEP pathway widely occurs in the bacterial kingdom and the plastids of plants, whereas the MVA pathway is commonly found in the cytosol of eukaryotes (2, 3). Thus, plants have the two different isoprenoid pathways in separate subcellular compartments. Recent work has shown that the majority of the prenyl side chain of iP and tZ is derived from the MEP pathway in Arabidopsis seedlings (4).
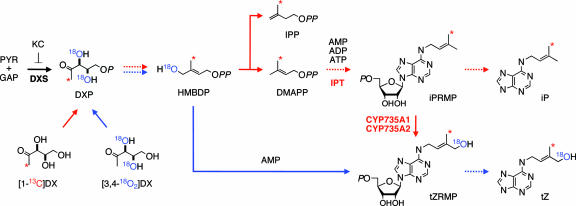
Fig. 1.
Schematic representation of isotope labeling of CK biosynthesis through the MEP pathway. Red arrows indicate the possible metabolism of [1-13C]DX (classical pathway). Blue arrows show possible conversions of [3,4-18O2]DX through HMBDP (iPRMP-independent pathway) into CKs. Dashed arrows denote multiple metabolic steps. 5-Ketoclomazone (KC) was included in all incubations to inhibit the endogenous MEP pathway. PYR, pyruvate; GAP, glyceraldehyde 3-phosphate; P, monophospholic; PP, diphospholic acid. CYP735A1 and CYP735A2 are cytochrome P450 monooxygenases in Arabidopsis (8).
To initiate CK biosynthesis, an isoprenoid precursor is transferred to AMP, ADP or ATP by adenosine phosphate-isopentenyltransferase (IPT, EC 2.5.1.27) (Fig. 1) (5–7). At least two routes have been proposed for the formation of tZ, an active CK, in plants. In the conventional iP riboside 5′-monophosphate (iPRMP)-dependent pathway, dimethylallyl diphosphate (DMAPP) is used as the side chain precursor for iPRMP, which is then converted to tZ riboside 5′-monophosphate (tZRMP) by P450 monooxygenases (8) (CYP735A1 and CYP735A2 in Arabidopsis; Fig. 1). The other proposed pathway is an iPRMP-independent bypass, in which an unidentified hydroxylated derivative of DMAPP is directly transferred to the adenine moiety (9). A candidate for this putative substrate is 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (HMBDP) (10), which has recently been shown to occur as an intermediate in the MEP pathway (11) (Fig. 1). However, the participation of HMBDP in CK biosynthesis has never been demonstrated in planta. It should also be noted that a fraction of tZ-type CKs would be produced in an iPRMP-independent manner through the isomerization of cis-zeatin (cZ)-type CKs, which are thought to be formed by the degradation of tRNA containing cZ-type prenylation (12), but the extent to which this occurs in Arabidopsis is unknown.
Tmr is an IPT that is encoded on the transferred-DNA (T-DNA) region of the Ti plasmid of Agrobacterium tumefaciens. Therefore, this bacterial enzyme functions in plant cells upon infection. Previous reports indicated that engineered expression of Tmr in plants elevated the synthesis of tZ-type CKs without a substantial increase in the production of iP derivatives (9, 13, 14). These observations imply that Tmr may be involved in the iPRMP-independent synthesis of tZ by using HMBDP in infected plant cells. In this model, however, it is unclear how Tmr recruits HMBDP as a substrate from the plastid-specific MEP pathway, because this bacterial protein does not have an apparent plastid-targeting sequence (see Fig. 6, which is published as supporting information on the PNAS web site).
To address how Tmr modifies CK biosynthesis in the host, we investigated the substrate preference and subcellular localization of Tmr in plant cells. Evidence is provided that Tmr is targeted to the plastid of infected plant cells and uses HMBDP in the MEP pathway as a major substrate. The plastid localization of Tmr explains how this bacterial enzyme utilizes the substrate in the plastid-localized isoprenoid pathway, but is surprising because it is a bacterial protein and does not have any apparent organelle-targeting sequence of plants.
Materials and Methods
Plant Materials. Arabidopsis thaliana ecotype Columbia was used in this study. A crown gall cell line of periwinkle V208 (Catharanthus roseus G. Don) (15) was cultured in hormone-free Murashige-Skoog (MS) liquid medium (16) supplemented with 3% sucrose. A wild-type cell line of periwinkle CRA was cultured in MS liquid medium supplemented with 3% sucrose and 0.5 μg/ml 2,4-dichlorophenoxyacetic acid. Both cell lines were provided by the Experimental Plant Division, RIKEN Bioresource Center (Tsukuba, Japan).
Chemicals. HMBDP was synthesized by Wako Pure Chemical (Osaka). [1-13C]1-deoxy-d-xylulose (DX) (99% labeled) was prepared as reported (4).
Synthesis of [3,4-18O2]DX. [3,4-18O2]DX (95% atom-labeled) was synthesized as reported (17) by using H218O (95% atom-labeled) for Sharpless asymmetric dihydroxylation of 1-benzyloxy-2(E)-penten-4-one. The structure of [3,4-18O2]DX was confirmed by its 1H and 13C NMR spectral data (18). To determine the 18O-incorporation into [3,4-18O2]DX, authentic DX and [3,4-18O2]DX were analyzed by gas chromatography-mass spectrometry after conversion to trimethylsilyl (TMS) derivatives as reported (19). [3,4-18O2]DX-TMS derivative was as follows: mass spectrum (EI, 70 eV) m/z: 311 (M-43+, 20%), 220 (100), 207 (50), 147 (70), 119 (40). DX-TMS derivative was as follows: mass spectrum (EI, 70 eV) m/z: 307 (M-43+, 20%), 218 (100), 205 (50), 147 (85), 117 (40).
Synthesis of 5-Ketoclomazone (KC). KC (20) was synthesized from 2-chlorophenylmethylhydroxylamine (21). To a solution of 2-chlorophenylmethylhydroxylamine (788 mg, 5 mmol) and triethylamine (1.01 g, 10 mmol) in CH2Cl2 (10 ml) was added dimethylmaronyl dichloride (845 mg, 5 mmol) in CH2Cl2 (2.5 ml) at 0°C. After the solution was stirred for 30 min, 1N-HCl was added to the reaction mixture, and then repeatedly extracted with ethyl acetate and then with CH2Cl2. The combined organic layers were washed with NaHCO3 solution, dried with Na2SO4, and concentrated. The product was purified by silica gel column chromatography to give KC (924 mg, yield 85%, colorless oil). The 1H NMR data of KC was identical with those of authentic sample (22).
Recombinant Enzymes. The coding region of Tmr from pTi-SAKURA (23) was ligated into pQE60 (Qiagen, Valencia, CA) to express the His-tagged recombinant proteins. Detailed procedures for preparation of the recombinant proteins have been described (6).
Transgenic Arabidopsis-Expressing IPT. The coding region of Tmr and AtIPTs was ligated into the pTA7001 vector (24) to yield a construct containing the dexamethasone-inducible IPT gene (pTA-Tmr, pTA-AtIPT1, pTA-AtIPT2, pTA-AtIPT3, pTA-AtIPT4, pTA-AtIPT5, or pTA-AtIPT7). The chimeric genes were introduced into Arabidopsis by the floral dip method (25). The T3 homozygous progenies were used for all experiments.
In Vivo Labeling Experiments. Seedlings of transgenic Arabidopsis plants were grown for 5 days on Murashige-Skoog (MS)-agar media and then transferred to MS liquid media (15 ml) containing KC (1 μM; added as a 15-μl methanol solution) and isotope-labeled DX (0.8 mM). Ten days later, seedlings were treated with 30 μM dexamethasone for 24 h, then harvested for CK analysis. For in vivo labeling, V208 and CRA cells were cultured with KC (1 μM) and isotope-labeled DX (0.8 mM) in a 100-ml flask for 1 week. Wild-type strains of A. tumefaciens MAFF302307 and MAFF302376 (native to Japan) were obtained from Ministry of Agriculture, Forestry and Fisheries GenBank at the National Institute of Agrobiological Sciences (Tsukuba, Japan). Colonies of wild-type Agrobacterium were inoculated onto wounded stem tissues of tomato (Lycopersicon esculentum Ailsa Craig) plants. When crown galls formed (4–6 weeks after incubation), they were injected with a mixture of 3 μl of KC (1 μM) and 30 μl of isotope-labeled DX (0.8 mM) by using a syringe. Galls were harvested 3 days after the injection for CK analysis. Extraction, purification, and analysis of CKs were performed as described (4). The 13C and 18O incorporation levels were calculated by using the molecular ion cluster ([M+H]+ and +1 to +4 isotopomers) after subtraction of natural 13C abundance (26).
CK Analysis. Extraction and determination of CKs from transgenic Arabidopsis lines overexpressing IPTs were performed as described (27).
Particle Bombardment. Full-length or partial coding regions of Tmr were fused to the amino terminus of the GFP gene, which was controlled by the cauliflower mosaic virus 35S promoter (35S–sGFP [S65T]) (28). As the control markers for plastids and mitochondria, signal peptides of Arabidopsis geranylgeranyl diphosphate synthase 1 and 6 (GGPS1 and GGPS6, respectively) (29) were fused to the amino terminus of GFP gene driven by the 35S promoter. The DNA constructs were introduced into roots of 2-week-old Arabidopsis seedlings by particle bombardment (PDU-1000/He, Bio-Rad). Transient expression was observed by laser confocal-scanning fluorescence microscopy after overnight incubation (Fluoview IX5, Olympus, Melville, NY).
Western Analysis. About 400 mg of seedlings were homogenized in two volumes of ice-cold extraction buffer (50 mM Tris·HCl, 150 mM NaCl, pH 7.5). Intact plastids were isolated from Arabidopsis seedlings and periwinkle cultured cells as described (30, 31). The proteins were subjected to SDS/PAGE followed by Western blotting.
Tmr Antibody. Polyclonal antibodies against Tmr were prepared with rabbits. The IgG was purified by the HiTrap Mab purification kit (Amersham Pharmacia Biosciences) and the method of epitope selection (32).
Results
Substrate Preference of IPTs. To investigate how the Agrobacterium Tmr modifies CK biosynthesis in plant cells, we first determined the Km values of Tmr for DMAPP and HMBDP by using the recombinant protein produced in Escherichia coli. Table 1 shows that Tmr transferred both DMAPP and HMBDP to AMP with similar Km values. This result suggests that Tmr may function in both the iPRMP-dependent and -independent pathways in Agrobacterium-infected plant cells. Unlike Tmr, recombinant AtIPT1 (a plastidic Arabidopsis IPT) did not use HMBDP as a substrate in combination with any of adenosine-phosphates (Table 1).
Table 1. The Km values of recombinant IPTs.
There are nine IPT-related sequences in the Arabidopsis genome (5, 6). Among them, AtIPT1, -3, -4, -5, -7, and -8 are likely to function as CK biosynthesis enzymes, because others are involved in the prenylation of tRNA (33), or do not encode a functional IPT in some Arabidopsis ecotypes (5). We have previously shown that AtIPT1, -3, -5, and -8 are localized to plastids when produced as fusion proteins with GFP in Arabidopsis cells, whereas AtIPT4-GFP and AtIPT7-GFP were detected in the cytosol and mitochondria, respectively (4).
Because it was difficult to obtain all AtIPTs as soluble recombinant proteins, we analyzed CK species accumulated in transgenic Arabidopsis lines overexpressing each IPT under the control of a dexamethasone-inducible promoter (24) to deduce the substrate preference. As reported, overexpression of Tmr in Arabidopsis resulted in accumulation of tZ-type CKs at extremely high levels without increasing the level of iP-type CKs substantially (Fig. 2; see Tables 2 and 3, which are published as supporting information on the PNAS web site) (9, 14). In contrast to the effect of Tmr overexpression, a large number of iP-type CKs accumulated in Arabidopsis seedlings when one of the native IPT genes (AtIPT1, -3, -4, -5, or -7) was overexpressed (Fig. 2). Predominant accumulation of iP nucleotides in AtIPT8-overexpressing Arabidopsis was also reported (34). Induction of AtIPT2 expression did not increase the level of any CK species because it catalyzes prenylation of tRNA (33). These results suggest that AtIPTs principally use DMAPP, but not HMBDP, for the biosynthesis of CKs in planta, regardless of their subcellular localization. In contrast, the major substrate for Tmr is likely to be HMBDP when overexpressed in Arabidopsis.
Fig. 2.
CK levels in transgenic Arabidopsis lines overexpressing IPTs. Transgenic Arabidopsis seedlings harboring pTA-Tmr, pTA-7001 (for vector control), pTA-AtIPT1, pTA-AtIPT2, pTA-AtIPT3, pTA-AtIPT4, pTA-AtIPT5, and pTA-AtIPT7 were grown for 18 days on MGRL-agar plates (42) and then subjected to dexamethasone (DEX) treatment. After 0, 6, and 12 h, the seedlings were harvested, and CK contents were measured. Only the results of iPRMP and tZRMP are presented. Results of other CK species from pTA-Tmr and pTA-7001 are shown in Tables 2 and 3. gFW, g fresh weight.
In Vivo Labeling Experiments. Although HMBDP has been a likely intermediate in the iPRMP-independent pathway and it does serve as a substrate for Tmr (Fig. 1, Fig. 2, and Table 1), there is no direct evidence that HMBDP is a precursor for CKs in any organism in vivo. To address this problem, we designed in vivo tracer experiments using [3,4-18O2]DX and [1-13C]DX (Fig. 1). From [3,4-18O2]DX, 18O would be incorporated through the MEP pathway into HMBDP, but not into DMAPP (Fig. 1; see Fig. 7, which is published as supporting information on the PNAS web site). On the other hand, [1-13C]DX would label both HMBDP and DMAPP with 13C (Fig. 1). Each of these labeled precursors was fed to Arabidopsis seedlings in the presence of KC, which blocks the endogenous MEP pathway (20, 35), to allow efficient labeling (Fig. 1).
To examine whether Tmr uses HMBDP in planta, we fed labeled DX to transgenic Arabidopsis plants expressing Tmr under the control of a dexamethasone-inducible promoter. Liquid chromatography-mass spectrometry analysis demonstrated that 18O was incorporated into tZ-type CKs from [3,4-18O2]DX when Tmr was overexpressed in Arabidopsis seedlings (Fig. 3A; see also Fig. 8, which is published as supporting information on the PNAS web site). However, only 13C from [1-13C]DX, but not 18O from [3,4-18O2]DX, was incorporated into tZ-derivatives without Tmr expression (Figs. 3A and 8). These results illustrate that Tmr uses HMBDP as a substrate in Arabidopsis seedlings, whereas this bypass does not occur at a detectable level without the engineered induction of Tmr in our experimental conditions. To further confirm this notion, we carried out in vivo labeling experiments using transgenic Arabidopsis plants overexpressing AtIPT1. As was the case with wild-type plants, only 13C from [1-13C]DX, but not 18O from [3, 4-18O2]DX, was incorporated into tZ nucleotides in the AtIPT1 overexpressor (Fig. 3B), supporting the idea that DMAPP (but not HMBDP) is the major substrate for AtIPT1 in Arabidopsis.
Fig. 3.
Incorporation of stable radioisotope-labeled DX into CKs. (A) Incorporation of [1-13C]DX or [3,4-18O2]DX into tZRMP in Arabidopsis seedlings with (pTA-Tmr) or without (pTA) overexpression of Tmr. (B) Incorporation of [3,4-18O2]DX or [1-13C]DX into tZRMP in Arabidopsis seedlings with (pTA-AtIPT1) or without (pTA) overexpression of AtIPT1.(C) Incorporation of [3,4-18O2]DX into tZRMP in cultured periwinkle cell lines V208 (crown gall) and CRA (wild-type). (D) A crown gall induced by inoculation of wild-type Agrobacterium on a tomato plant. [3,4-18O2]DX and KC were injected into the gall, and the 18O incorporation into tZRMP was analyzed qualitatively (see In Vivo Labeling Experiments). Values in A and C are means with SD (n = 3).
To investigate whether HMBDP is used for tZ synthesis in plants upon Agrobacterium infection, CK biosynthesis in a cultured crown gall cell line of periwinkle (V208) (15) was examined. V208 cells contain an integration of the Agrobacterium T-DNA, but they are free of bacteria unlike natural crown galls. Liquid chromatography-mass spectrometry analysis showed that a large fraction of tZ-type CKs was labeled with 18O when [3,4-18O2]DX was fed in the presence of KC (Fig. 3C). A high incorporation rate of [3,4-18O2]DX into tZRMP indicates that HMBDP is the major source of prenyl side chain of tZ derivatives in the V208 crown gall cells. In contrast, 18O incorporation was not observed in another periwinkle cell line, CRA, which does not possess a T-DNA integration (Fig. 3C). To examine the occurrence of the HMBDP pathway in natural crown galls, we inoculated wild-type Agrobacterium onto tomato plants. The resulting galls were injected with [3,4-18O2]DX (Fig. 3D). From galls induced by two independent Agrobacterium isolates, we were able to detect 18O incorporation (34% and 59%) into tZRMP, indicating that the HMBDP pathway operates in crown galls in nature.
Subcellular Localization of Tmr in Arabidopsis. Because HMBDP is a metabolic intermediate in the MEP pathway, Tmr should exist in plastids to use HMBDP as a substrate. To determine the subcellular localization of Tmr, a series of Tmr-GFP fusion genes were transiently expressed in Arabidopsis root cells (Fig. 4 A–G). As the control markers for plastids and mitochondria, Arabidopsis geranylgeranyl diphosphate synthase 1 and 6 (29) were used, respectively (Fig. 4 H and I). Consistent with the use of HMBDP by Tmr in the MEP pathway, fluorescence from the full-length Tmr-GFP was localized to plastids (Fig. 4A). Deletion analyses showed that the amino-terminal 124 aa were sufficient to drive the fusion protein to plastids (Fig. 4 B–D). A removal of 60 aa at the amino terminus was found to abolish the plastid localization (Fig. 4E). This region seems to be necessary for both the catalysis and the plastid translocation, because it contains a putative P-loop motif (36) and a conserved aspartate residue (37) essential for the activity. When GFP was fused to the amino terminus of Tmr, the fusion protein did not localize to the plastid (Fig. 4F), suggesting the necessity of the intact amino terminus for its plastid localization.
Fig. 4.
Plastid localization of Tmr in transgenic Arabidopsis seedlings. A series of fusion genes [Tmr-GFP (A), Tmr60ΔC-GFP (B), Tmr124ΔC-GFP (C), Tmr177ΔC-GFP (D), Tmr60ΔN-GFP (E), GFP-Tmr (F), GFP (G), GGPS1-GFP (H), and GGPS6-GFP (I)] were transiently expressed in Arabidopsis root cells by particle bombardment. (Scale bar: 20 μm.) (J) Schematic representation of the fusion constructs. (K) Western blot analysis of total leaf proteins (Total) and the chloroplast fraction (Chl.) (equivalent to 1.3 μg of chlorophyll) using antibodies against Tmr or glutamine synthetase (GS). Proteins were extracted from transgenic Arabidopsis seedlings harboring pTA-Tmr with (+) or without (–) dexamethasone (DEX) treatment for 24 h. GS1 and GS2, cytosolic and plastidic GS, respectively.
Anti-Tmr antibodies were used to analyze subcellular localization of Tmr in protein extracts from stable transformants expressing Tmr. Immunoblot analysis showed that Tmr was detected in a chloroplast-enriched fraction that is marked by the presence and absence of plastid- and cytosol-localized glutamine synthetase isoforms, respectively (Fig. 4K).
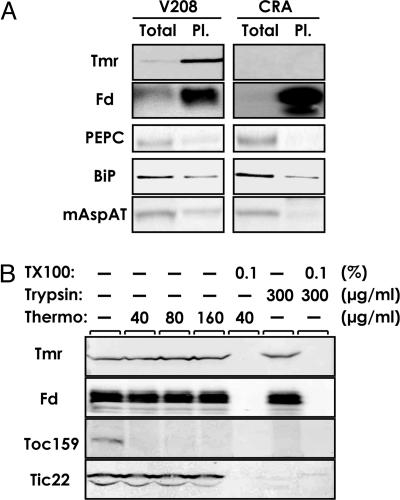
Subcellular Localization of Tmr in Crown Gall Cells. To examine whether Tmr is localized to plastids in crown galls, intact plastids were purified from the cell line V208 and CRA of periwinkle. Tmr was enriched in the plastid fraction relative to the total cell extract, as was the case with ferredoxin used as a plastid marker (Fig. 5A). We confirmed that marker proteins for the cytosol (phosphoenolpyruvate carboxylase), endoplasmic reticulum (binding protein, BiP), and mitochondria (mitochondrial aspartate aminotransferase) were not enriched in the plastid fraction (Fig. 5A). To assess suborganellar localization of Tmr in the V208 cells, isolated plastids were exposed to trypsin or thermolysin (Fig. 5B). These proteinases have different penetrability to plastid envelopes and are useful to assess the location of plastidial proteins (30). Unlike envelope marker proteins that were sensitive to trypsin (Tic22) or both trypsin and thermolysin (Toc159), Tmr and ferredoxin (a marker for stroma) were resistant to these proteases, but were sensitive to Triton X-100, which destroys the organelle membrane structure (Fig. 5B). These data indicate that Tmr is localized to the stroma of plastids in crown gall cells.
Fig. 5.
Plastid localization of Tmr in crown gall cells. (A) Proteins of total cell extract (Total) and intact plastid fraction (Pl.) (equivalent to 3 μg of protein), prepared from periwinkle V208 or CRA cells, were analyzed by Western blotting. Fd, ferredoxin, a soluble stromal marker; PEPC, phosphoenolpyruvate carboxylase, a cytosolic marker; BiP, binding protein, an endoplasmic reticulum marker; mAspAT, mitochondrial aspartate aminotransferase, a mitochondrial marker. (B) Intact plastids from V208 cells were treated with the indicated concentration of thermolysin (Thermo) on the ice or trypsin (Trypsin) at 25°C for 30 min with or without 0.1% Triton X-100 (TX100). Total proteins from the isolated plastid after respective treatments were then analyzed by Western blotting. Toc159 and Tic22, plastid outer and inner envelope markers, respectively.
Discussion
In this study, we have demonstrated that Agrobacterium modifies CK biosynthesis by sending a key enzyme into plastids to promote tumorigenesis. In uninfected plants, the ratio of auxin and CK, both of which are targets of Agrobacterium for tumorigenesis, is carefully regulated. Recent work has indicated that expression of CYP735A genes is negatively regulated by auxin (8), which suggests that auxin limits the level of tZ in plants. Because elevated synthesis of both auxin and CK is essential for tumorigenesis, the use of HMBDP for tZ biosynthesis without the catalysis by CYP735As may play an important role in circumventing the hormonal homeostasis in plants. Although tZ has generally been thought as the most active CK (38, 39), it has not been entirely clear whether only tZ is active in inducing the tumor formation or whether iP also acts as an active form. Recently, we found that Arabidopsis plants do not grow normally when CYP735A genes are knocked out (K.T. and H.S., unpublished results), suggesting that tZ plays a distinguishable role from iP in plant development, and perhaps in tumorigenesis.
Although Tmr uses both DMAPP and HMBDP with similar Km values in vitro (Table 1), isotope tracer experiments indicated that the major substrate for Tmr was HMBDP, but not DMAPP, in crown gall cells (Fig. 3C). Because HMBDP reductase produces isopentenyl diphosphate and DMAPP at a ratio of 5:1 (40), the available pool size of DMAPP for IPTs may be relatively smaller than that of HMBDP in plastids. This finding would explain why tZ-type CKs are predominantly produced in Agrobacterium-infected plant cells.
The iPRMP-independent pathway has initially been proposed in Tmr-overexpressing Arabidopsis plants, based on biased synthetic rates between iPRMP and tZRMP by using 2H2O/[2H6]isopentenyladenine riboside double tracers (9). In this pioneer work, it was indicated that the iPRMP-independent synthesis of tZRMP occurs also in wild-type Arabidopsis seedlings, when hydroxylation of iPRMP was blocked by metyrapone, an inhibitor for CYP735As (9). In the present study, the incorporation of [3,4-18O2]DX into tZ-type CKs depended on expression of Tmr; the majority of tZ derivatives were labeled with 13C from [1-13C]DX, but not with 18O from [3,4-18O2]DX, in control (no Tmr expression) and AtIPT1-overexpressing Arabidopsis plants (Fig. 3 A and B). In addition, only Tmr, but none of AtIPTs, caused dominant accumulation of tZ-type CK when overproduced in Arabidopsis (Fig. 2). These results indicate that the major isoprenoid precursor for tZ biosynthesis is DMAPP, but not HMBDP, in wild-type Arabidopsis seedlings, but that the HMBDP bypass through the MEP pathway is newly created upon expression of Tmr. In this context, the identification of the iPRMP-independent tZRMP synthesis in metyrapone-treated wild-type Arabidopsis (9, 41) is unexplained; it was suggested that the isoprenoid moiety of tZRMP in treated plants came from the MVA pathway. We speculate that a fraction of tZ-type CKs in wild-type Arabidopsis might be synthesized in an iPRMP-independent manner by means of cis-trans isomerization of cis-zeatin derivatives, of which the prenyl side chain is primarily derived from the MVA pathway in Arabidopsis seedlings (9). However, the occurrence of the cis-trans isomerization of zeatin has not been demonstrated in Arabidopsis. It is therefore premature to rule out the presence of the HMBDP pathway in metyrapone-treated wild-type Arabidopsis.
The plastid targeting of Tmr is likely to be achieved by the host import machinery, because Tmr on its own was delivered to this organelle in the absence of any other Agrobacterium protein (Fig. 4). How Tmr moves into the organelle without an apparent plastid-import sequence requires further investigation. It is intriguing that the bacterium protein Tmr functions in the plastid, an organelle that has symbiotically evolved from a bacterial ancestor. The MEP pathway is widely found in prokaryotic systems (3), including Agrobacterium, suggesting that Tmr has coevolved with the MEP pathway, but not with the MVA pathway. It seems a logical strategy that Tmr produced in the plant cell is designed to function in the plastid, which provides the key substrate HMBDP through the prokaryotic isoprenoid pathway.
Supplementary Material
Acknowledgments
We thank Dr. Damian O'Neill (RIKEN) and Dr. Peter McCourt (University of Toronto, Toronto) for their critical reading of this manuscript. We also thank Drs. T. Hase and M. Nakai (Osaka University, Osaka) for providing us with antibodies against maize ferredoxins and Arabidopsis Toc159 and Tic22, Dr. I. Hara-Nishimura (Kyoto University, Kyoto) for antibody against pumpkin BiP, Dr. M. Taniguchi (Nagoya University, Nagoya, Japan) for mitochondrial aspartate aminotransferase, and Dr. N.-H. Chua (The Rockefeller University, New York) for providing pTA-7001. This study was partly supported by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.
Author contributions: H.S., H.K., and S.Y. designed research; H.S., H.K., N.U., and M.K. performed research; H.S., H.K., K.T., Y.K., T.Y., and S.Y. analyzed data; S.H., T.A., and K.O. contributed new reagents/analytic tools; and H.S., H.K., and S.Y. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CK, cytokinin; DMAPP, dimethylallyl diphosphate; DX, 1-deoxy-d-xylulose; HMBDP, 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate; iP, isopentenyladenine; iPRMP, isopentenyladenine riboside 5′-monophosphate; IPT, adenosine phosphates-isopentenyltransferase; KC, 5-ketoclomazone; MEP, methylerythritol phosphate; MVA, mevalonate; tZ, trans-zeatin; tZRMP, trans-zeatin riboside 5′-monophosphate.
References
- 1.Mok, D. W. & Mok, M. C. (2001) Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 89–118. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenthaler, H. K. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 47–65. [DOI] [PubMed] [Google Scholar]
- 3.Rohmer, M. (1999) Nat. Prod. Rep. 16, 565–574. [DOI] [PubMed] [Google Scholar]
- 4.Kasahara, H., Takei, K., Ueda, N., Hishiyama, S., Yamaya, T., Kamiya, Y., Yamaguchi, S. & Sakakibara, H. (2004) J. Biol. Chem. 279, 14049–14054. [DOI] [PubMed] [Google Scholar]
- 5.Kakimoto, T. (2001) Plant Cell Physiol. 42, 677–685. [DOI] [PubMed] [Google Scholar]
- 6.Takei, K., Sakakibara, H. & Sugiyama, T. (2001) J. Biol. Chem. 276, 26405–26410. [DOI] [PubMed] [Google Scholar]
- 7.Sakakibara, H. (2005) in Plant Hormones: Biosynthesis, Signal Transduction, Action!, ed. Davies, P. J. (Springer, Dordrecht, The Netherlands), pp. 95–114.
- 8.Takei, K., Yamaya, T. & Sakakibara, H. (2004) J. Biol. Chem. 279, 41866–41872. [DOI] [PubMed] [Google Scholar]
- 9.Åstot, C., Dolezal, K., Nordström, A., Wang, Q., Kunkel, T., Moritz, T., Chua, N.-H. & Sandberg, G. (2000) Proc. Natl. Acad. Sci. USA 97, 14778–14783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krall, L., Raschke, M., Zenk, M. H. & Baron, C. (2002) FEBS Lett. 527, 315–318. [DOI] [PubMed] [Google Scholar]
- 11.Hecht, S., Eisenreich, W., Adam, P., Amslinger, S., Kis, K., Bacher, A., Arigoni, D. & Rohdich, F. (2001) Proc. Natl. Acad. Sci. USA 98, 14837–14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murai, N. (1994) in Cytokinins: Chemistry, Activity, and Function, eds. Mok, D. W. S. & Mok, M. C. (CRC, Boca Raton, FL), pp. 87–99.
- 13.Stuchbury, T., Palni, L. M. S., Horgan, R. & Wareing, P. F. (1979) Planta 147, 97–102. [DOI] [PubMed] [Google Scholar]
- 14.Faiss, M., Zalubilova, J., Strnad, M. & Schmülling, T. (1997) Plant J. 12, 401–415. [DOI] [PubMed] [Google Scholar]
- 15.Park, K. H., Saimoto, H., Nakagawa, S., Sakurai, A., Yokota, T., Takahashi, N. & Syono, K. (1989) Agric. Biol. Chem. 53, 805–811. [Google Scholar]
- 16.Murashige, T. & Skoog, F. (1962) Physiol. Plant. 15, 473–497. [Google Scholar]
- 17.Giner, J.-L. (1998) Tetrahedron Lett. 39, 2479–2482. [Google Scholar]
- 18.Blagg, B. S. & Poulter, C. D. (1999) J. Org. Chem. 64, 1508–1511. [DOI] [PubMed] [Google Scholar]
- 19.Lange, B. M., Wildung, M. R., McCaskill, D. & Croteau, R. (1998) Proc. Natl. Acad. Sci. USA 95, 2100–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ElNaggar, S. F., Creekmore, R. W., Schocken, M. J., Rosen, R. T. & Robinson, R. A. (1992) J. Agric. Food Chem. 40, 880–883. [Google Scholar]
- 21.Chang, J. H. (1983) U.S. Patent 4,405,357.
- 22.Seki, E., Harada, T., Masutani, T. & Kitahara, K. (1988) Japan Patent JP 1986-278129.
- 23.Suzuki, K., Hattori, Y., Uraji, M., Ohta, N., Iwata, K., Murata, K., Kato, A. & Yoshida, K. (2000) Gene 242, 331–336. [DOI] [PubMed] [Google Scholar]
- 24.Aoyama, T. & Chua, N.-H. (1997) Plant J. 11, 605–612. [DOI] [PubMed] [Google Scholar]
- 25.Clough, S. J. & Bent, A. F. (1998) Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- 26.Rauschenbach, P., Schmidt, H. L., Simon, H., Tykva, R. & Wenzel, M. (1974) Messung von Radioaktiven und Stabilen Isotopen (Springer, Heidelberg).
- 27.Nakagawa, H., Jiang, C.-J., Sakakibara, H., Kojima, M., Honda, I., Ajisaka, H., Nishijima, T., Koshioka, M., Homma, T., Mander, L. N. & Takatsuji, H. (2005) Plant J. 41, 512–523. [DOI] [PubMed] [Google Scholar]
- 28.Niwa, Y., Hirano, T., Yoshimoto, K., Shimizu, M. & Kobayashi, H. (1999) Plant J. 18, 455–463. [DOI] [PubMed] [Google Scholar]
- 29.Okada, K., Saito, T., Nakagawa, T., Kawamukai, M. & Kamiya, Y. (2000) Plant Physiol. 122, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson, D. T., Froehlich, J. E. & Keegstra, K. (1998) J. Biol. Chem. 273, 16583–16588. [DOI] [PubMed] [Google Scholar]
- 31.Sakai, A., Suzuki, T., Miyazawa, Y. & Kuroiwa, T. (1998) Plant Sci. 133, 17–31. [Google Scholar]
- 32.Sakakibara, H., Watanabe, M., Hase, T. & Sugiyama, T. (1991) J. Biol. Chem. 266, 2028–2035. [PubMed] [Google Scholar]
- 33.Golovko, A., Sitbon, F., Tillberg, E. & Nicander, B. (2002) Plant Mol. Biol. 49, 161–169. [DOI] [PubMed] [Google Scholar]
- 34.Sun, J., Niu, Q. W., Tarkowski, P., Zheng, B., Tarkowska, D., Sandberg, G., Chua, N. H. & Zuo, J. (2003) Plant Physiol. 131, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeidler, J., Schwender, J., Mueller, C. & Lichtenthaler, H. K. (2000) Biochem. Soc. Trans. 28, 796–798. [PubMed] [Google Scholar]
- 36.Leung, H.-C. E., Chen, Y. & Winkler, M. E. (1997) J. Biol. Chem. 272, 13073–13083. [DOI] [PubMed] [Google Scholar]
- 37.Sakano, Y., Okada, Y., Matsunaga, A., Suwama, T., Kaneko, T., Ito, K., Noguchi, H. & Abe, I. (2004) Phytochemistry 65, 2439–2446. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz, R. Y. & Skoog, F. (1972) Plant Physiol. 50, 702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiess, L. D. (1975) Plant Physiol. 55, 583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohdich, F., Hecht, S., Gartner, K., Adam, P., Krieger, C., Amslinger, S., Arigoni, D., Bacher, A. & Eisenreich, W. (2002) Proc. Natl. Acad. Sci. USA 99, 1158–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nordström, A., Tarkowski, P., Tarkowska, D., Norbaek, R., Åstot, C., Dolezal, K. & Sandberg, G. (2004) Proc. Natl. Acad. Sci. USA 101, 8039–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiwara, T., Yokota-Hirai, M., Chino, M., Komeda, Y. & Naito, S. (1992) Plant Physiol. 99, 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.