Abstract
The protozoan parasite Toxoplasma gondii relies on calcium-mediated exocytosis to secrete adhesins on to its surface where they can engage host cell receptors. Increases in intracellular calcium occur in response to Ins(1,4,5)P3 and caffeine, an agonist of ryanodine-responsive calcium-release channels. We examined lysates and microsomes of T. gondii and detected evidence of cADPR (cyclic ADP ribose) cyclase and hydrolase activities, the two enzymes that control the second messenger cADPR, which causes calcium release from RyR (ryanodine receptor). We also detected endogenous levels of cADPR in extracts of T. gondii. Furthermore, T. gondii microsomes that were loaded with 45Ca2+ released calcium when treated with cADPR, and the RyR antagonists 8-bromo-cADPR and Ruthenium Red blocked this response. Although T. gondii microsomes also responded to Ins(1,4,5)P3, the inhibition profiles of these calcium-release channels were mutually exclusive. The RyR antagonists 8-bromo-cADPR and dantrolene inhibited protein secretion and motility in live parasites. These results indicate that RyR calcium-release channels that respond to the second-messenger cADPR play an important role in regulating intracellular Ca2+, and hence host cell invasion, in protozoan parasites.
Keywords: calcium channel, cyclic ADP ribose (cADPR), microneme secretion, parasite, signalling
Abbreviations: cADPR, cyclic ADP ribose; 8-Br-cADPR, 8-bromo-cADPR; ER, endoplasmic reticulum; FBS, foetal bovine serum; IP3, Ins(1,4,5)P3; IP3R, IP3 receptor; NGD, nicotinamide guanine dinucleotide; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite that infects all types of nucleated cells in a wide range of warm-blooded vertebrates [1]. Cellular invasion occurs by an active process that relies on the actin–myosin cytoskeleton of the parasite to drive entry into the host cells [2,3]. Entry is also very much dependent on the controlled release of adhesins from apical secretory organelles called micronemes, which harbour a collection of proteins that bear distinct adhesive domains [4]. Microneme secretion occurs at the extreme apex of the parasite and is thought to be responsible for the polarized attachment to host cells [5].
Microneme secretion is a calcium-mediated event and sequestration of intracellular calcium with BAPTA/AM [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid acetoxymethyl ester] results in the inhibition of secretion and decreased attachment [6]. Conversely, increase of intracellular calcium with ionophores results in rapid secretion of microneme proteins [6]. Agonists that stimulate rapid secretion, such as calcium ionophores or ethanol [7], uncouple secretion from apical attachment and consequently decrease motility and invasion by the parasite. Insight into the calcium-mediated motility has also been provided using the compound calmidazolium, which in most systems is a potent inhibitor of calmodulin. Similar to Dictyostelium, this compound acts as a calcium agonist in T. gondii, and treatment with low micromolar doses results in sustained calcium oscillations that increase secretion and thereby prolong gliding and also increase cellular invasion by the parasite [8]. Collectively, these findings indicate that oscillations in intracellular calcium levels govern microneme secretion and hence motility in T. gondii.
Intracellular calcium is regulated by two primary pathways in eukaryotic cells [9]. In response to engagement of receptor tyrosine kinase or heterotrimeric G-protein-coupled cell-surface receptors, generation of IP3 [Ins(1,4,5)P3] acts on calcium channels in the ER (endoplasmic reticulum) to release calcium into the cytosol. A separate pathway that operates in various cell types is governed by the intracellular messenger cADPR (cyclic ADP ribose), the product of a specific cyclase [10]. cADPR is an endogenous regulator of the ryanodine channels [RyR (ryanodine receptor)], which are also stimulated by caffeine and the plant alkaloid ryanodine, resulting in release of calcium from ER stores [11]. In vertebrates, IP3Rs (IP3 receptors) are generally expressed in all cells, whereas RyRs are typically confined to neuromuscular and specialized secretory cells [9]. However, T-lymphocytes [12] and some non-excitable cells also express some isoforms of RyR [13]. Ryanodine-responsive channels are also known in invertebrates, including Drosophila [14] and sea-urchin eggs [15]. Additionally, cADPR-induced calcium fluxes occur in Hydra [16], sponges [17] and plants [18], suggesting an ancient origin for this signalling pathway.
Intracellular calcium plays an important role in differentiation [19], motility [8,20], cytoskeletal dynamics [21] and cell growth [22,23] in protozoan parasites. In addition to the normal intracellular calcium storage pools in the ER and mitochondria, protozoa also contain a unique intracellular organelle for calcium storage called the acidocalcisome [24,25]. Acidocalcisomes do not appear to play a role in the rapid calcium signalling process, but rather serve as a sink for calcium and are probably also important sites for polyphosphate metabolism [24,25]. Previous studies have demonstrated that intracellular calcium in T. gondii is responsible for controlling secretion, motility and cell invasion [26]. In contrast, extracellular calcium plays little direct role and calcium levels in the host cell have no effect on parasite invasion. Two separate response pathways have been inferred by pharmacological studies in T. gondii [27]. First, treatment with ethanol increases intracellular calcium, and this pathway is sensitive to inhibitors of IP3 channels. T. gondii also responds to agonists of cADPR-gated channels such as ryanodine and caffeine [27]. Caffeine also stimulates calcium release from intracellular pools in ciliates, leading to exocytosis [28,29]. However, the molecular targets of caffeine or ryanodine remain unknown in protozoa and, despite pharmacological evidence for their existence, intracellular calcium channels of the IP3R/RyR families have not been identified at the gene or protein level.
In the present study, we explore the hypothesis that protozoa contain calcium-release channels using the model parasite T. gondii. Our studies provide biochemical evidence for a cADPR-gated calcium channel controlling microneme protein secretion and motility in T. gondii.
EXPERIMENTAL
Materials
Lytechinus pictus (sea urchin) were obtained from Marinus (Long Beach, CA, U.S.A.). Fluo-3 was purchased from Molecular Probes (Eugene, OR, U.S.A.), and IP3, ryanodine, oligomycin and antimycin were from Calbiochem (San Diego, CA, U.S.A.). 8-Br-cADPR (8-bromo-cADPR), dantrolene, heparin and other reagents, of the highest purity grade available, were supplied by Sigma (St. Louis, MO, U.S.A.).
Parasite and cell cultures
RH strain T. gondii were propagated as tachyzoites in monolayers of human fibroblasts as described previously [26]. Parasites were harvested after natural egress and then separated by filtration through 3 μm polycarbonate membranes followed by centrifugation at 400 g for 10 min. Cells were resuspended in Hanks balanced salt solution containing 0.1 mM EGTA and 10 mM Hepes (pH 7.2).
MIC2 secretion assay
Purified parasites were treated with different concentrations of dantrolene, 8-Br-cADPR or DMSO for 1, 6 or 12 min at 4 °C on wet ice. Secretion was stimulated by transferring the samples to 37 °C for 5 min followed by returning them to ice. After the stimulation, samples were divided into the supernatant and cell pellet by centrifugation at 400 g for 10 min at 4 °C. Proteins were separated by SDS/PAGE and transferred on to nitrocellulose membranes. Western blotting was performed using rabbit anti-MIC2 antibody (1:10000) and horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10000; Jackson Immunoresearch Laboratories, West Grove, PA, U.S.A.). Signals were detected using Super Signal West Pico (Pierce, Rockford, IL, U.S.A.) for qualitative analysis and ECL® plus Western blotting detection system (Amersham Biosciences, Little Chalfont, Bucks., U.K.) for quantitative analysis. PhosphoImager analysis was performed using a Fuji FLA-5000 and Image Gauge v.4.0 (Fuji Film, Tokyo, Japan) and the results were averaged from three separate experiments.
Trail gliding assay
Parasites were treated with antagonists for 15 min on ice, transferred to LabTek (Nalge Nunc International, Naperville, IL, U.S.A.) glass chamber slides that had been precoated with FBS (foetal bovine serum) and incubated for 20 min at 37 °C. After the incubation, cells were fixed with 4% (w/v) paraformaldehyde, permeabilized with 0.05% saponin and stained with anti-SAG1 monoclonal antibody (DE52) as described previously [8]. The stained samples were observed using a Zeiss Axioscope microscope equipped for epifluorescence illumination, and the average number and length of trails for a high-powered (×63) field from triplicate samples were determined. The results are expressed as the average of three independent experiments.
Video microscopy
Glass bottom dishes (MatTek, Ashland, MA, U.S.A.) were coated with 100% (v/v) FBS for 30 min. Parasites were loaded with 1 μM Fluo-4 AM (Molecular Probes) for 5 min at 37 °C, centrifuged and then resuspended in warm Ringer's buffer (155 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 3 mM NaH2PO4, 10 mM Hepes and 10 mM glucose) containing 1% FBS+0.02% DMSO, 1 μM 8-Br-cADPR or 1 μM dantrolene, and added to dishes. Alternating time-lapse phase and fluorescent images were collected at one frame/1.5 s under low-light illumination on a Zeiss upright Axioskope (Zeiss, Cologne, Germany) over a 15 min time period using a Hamamatsu ORCA ER camera (Hamamatsu, Japan) operated with Openlabs version 4.0.1 (Improvision, Lexington, MA, U.S.A.).
The average duration of gliding was calculated using five random fields containing at least ten parasites for each condition. Fluo-4 analysis was based on previously defined parameters for the ‘average duration’ (time between the start of successive cycles), ‘peak-to-low’ (time between the highest signal and the lowest signal during a cycle), and ‘time between’ (time between successive peaks) as described previously [8,26]. The average values for each experiment were calculated for three or more parasites per condition from three separate experiments.
T. gondii homogenates
Cells were resuspended in a medium containing 0.25 M sucrose, 20 mM Tris/HCl (pH 7.2) and 20 μg/ml leupeptin. Parasites were sonicated, and the homogenized cells were centrifuged at 2000 g for 10 min at 4 °C. The supernatants were collected and used for the determination of enzymatic synthesis of cADPR.
Synthesis and hydrolysis of cADPR
ADP-ribosyl cyclase activity was assayed using NAD+ as described previously [30]. The T. gondii homogenates were incubated at 1 mg/ml in a medium containing 1 mM NAD, 0.25 M sucrose and 40 mM Tris/HCl (pH 7.2) at 37 °C. Aliquots before and after the incubation were assayed for cADPR content using the seaurchin egg homogenate bioassay and HPLC as described previously [30]. Specific activity was expressed as nmol of cADPR produced/mg of protein. Activity of the ADP-ribosyl cyclase was also performed using the NGD (nicotinamide guanine dinucleotide) technique as described previously [31]. Enzyme preparations were incubated in a medium containing 0.2 mM NGD, 0.25 M sucrose and 40 mM Tris/HCl (pH 7.2) at 37 °C. Activity was measured by a fluorimetric assay at 300 nm excitation and 410 nm emission. Hydrolysis of cADPR was determined using the sea-urchin egg homogenate bioassay [32].
HPLC analysis of nucleotides
The synthesis of cADPR by T. gondii extracts was verified by HPLC anion-exchange chromatography using an AG MP-1 (Bio-Rad Laboratories, Hercules, CA, U.S.A.) column eluted with a nonlinear gradient of trifluoroacetic acid as described previously [30,32]. The nucleotides were detected by UV absorption at 254 nm. The authenticity of the cADPR produced was confirmed by co-elution with standard compounds and by the sea-urchin egg bioassay as described previously [30,32].
Synthesis of standard nucleotides
cADPR was synthesized by Aplysia ADP-ribosyl cyclase, using homogenized Aplysia ovotestes as described previously [30,32]. The reaction was stopped by acetone precipitation as described above for the synthesis of cADPR.
After acetone precipitation, the nucleotides were purified by HPLC anion-exchange chromatography using AG MP-1 (Bio-Rad Laboratories) resin packed into a 1 cm×10 cm column. The nucleotides were eluted with a nonlinear gradient of 150 mM trifluoroacetic acid and water, and monitored by UV absorption at 250 nm. Purified cADPR was evaporated to dryness in a SpeedVac concentrator. The cADPR used in all experiments was at least 97% pure as determined by HPLC.
Detection of cADPR in T. gondii
Nucleotides were extracted from T. gondii cells with 5% (v/v) trichloroacetic acid at 4 °C, followed by removal with water-saturated diethyl ether as described previously [33]. The aqueous layer containing the cADPR was adjusted to pH 8 with 20 mM sodium phosphate. To remove nucleotides other than cADPR, a mixture containing hydrolytic enzymes was added to the samples, with the following final concentrations: 0.44 unit/ml nucleotide pyrophosphatase, 12.5 units/ml alkaline phosphatase, 0.0625 unit/ml NADase, 2.5 mM MgCl2 and 20 mM sodium phosphate (pH 8.0). Incubation proceeded overnight at 37 °C. Detection of cADPR was performed with some modifications to the cycling method described recently [33,34]. In brief, 0.1 ml of cADPR standard or nucleotides extracted from T. gondii samples were incubated with 1000 μl of cycling reagent containing 0.3 μg/ml ADP-ribosyl cyclase, 4 mM nicotinamide, 100 mM sodium phosphate (pH 8), 2% (v/v) ethanol, 40 μg/ml alcohol dehydrogenase, 8 μM resazurin, 0.04 μg/ml diaphorase and 4 μM flavin mononucleotide. Increase in the resorufin fluorescence (with excitation at 544 nm and emission at 590 nm) was measured using a Hitachi F-2000 fluorimeter. The results are expressed as means±S.E.M. from at least three independent measurements. The recovery rate of exogenous cADPR detected by this method, after trichloroacetic acid extraction of the standard concentrations of cADPR, was in the range 75–80%.
Microsomal fractionation
Microsomal fractions were prepared by the method described previously [34]. T. gondii were homogenized in 30% (w/v) homogenizing buffer containing 0.25 M sucrose and 40 mM Tris/HCl (pH 7.2). The cells were homogenized with a glass-Teflon homogenizer at 1500 rev./min using four to six strokes. The homogenates were centrifuged at 660 g for 15 min and the resulting supernatant was centrifuged at 6800 g for 15 min. The resulting supernatant was further centrifuged at 20000 g for 20 min and the supernatant was collected and centrifuged at 100000 g for 60 min in an ultracentrifuge. The pellet of the last centrifugation was resuspended in a medium containing 0.25 M sucrose and 40 mM Tris/HCl (pH 7.2), and is denoted hereinafter as the microsomal fraction.
45Ca2+ uptake and release
Microsomes from T. gondii were prepared as described above. Fresh isolated microsomes were incubated at 37 °C in a water bath and diluted to 1.0 mg/ml with an intracellular medium containing 250 mM N-methyl glucamine, 250 mM potassium gluconate, 20 mM Hepes buffer (pH 7.2), 1 mM MgCl2, 2 units/ml creatine kinase, 4 mM phosphocreatine, 1 mM ATP, 3 μg/ml oligomycin and 3 μg/ml antimycin, in the presence or absence of thapsigargin. Microsomes were incubated with 45Ca at 5000 c.p.m./nmol of Ca2+ for 30 min at 4 °C, after which they maintained a stable amount of intravesicular Ca2+. 45Ca uptake and release were measured by a filtration method using glass-fibre filters as described previously [35]. The remaining intravesicular 45Ca was determined by filtration of 0.2 ml of 1.0 mg/ml T. gondii microsomes through a prewashed GF/C glass fibre filter (Whatman Biosystems, Maidstone, Kent, U.K.) under vacuum, followed by three rapid washes with 1 ml of an ice-cold intracellular medium containing 3 mM LaCl3. The radioactivity retained on the filter was determined by standard scintillation counting.
Ryanodine binding
[3H]Ryanodine binding was performed using a filtration method described previously [35]. In brief, T. gondii microsomal fractions (100–200 μg of protein) were incubated in a medium containing (final concentrations) 600 mM KCl, 100 μM EGTA, 0.2 mM PMSF, 25 mM Hepes (pH 7.2) and saturating concentrations of [3H]ryanodine (180 nM) for 30 min at 35 °C. The reaction was stopped by applying the sample to a GF/C glass fibre filter and washing under vacuum three times with ice-cold water. The radioactivity remaining in the filter was determined with standard scintillation counting techniques.
Statistics
The reported experiments were repeated three to six times. Statistical comparisons were performed using Student's t test. Values are reported as means±S.D.
RESULTS
Evidence that T. gondii contains an endogenous cADPR pathway
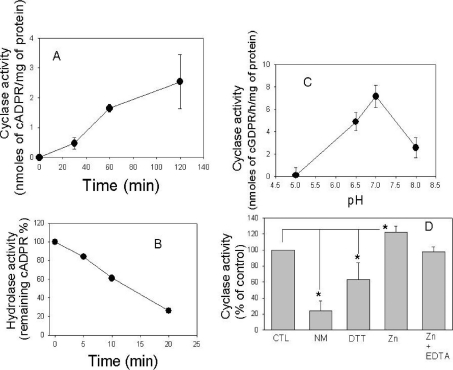
Previous studies have suggested the presence of RyR-type channels in T. gondii on the basis of increases in intracellular calcium in parasites treated with ryanodine or caffeine [27]. One natural agonist of ryanodine channels is cADPR, a product of cADPR cyclase [11]. Therefore we examined extracts of T. gondii for cADPR cyclase activity using the substrate NAD. cADPR was monitored using the sea-urchin egg homogenate bioassay and confirmed by HPLC as described previously [30]. Increasing concentrations of cADPR were detected in lysates of the parasite incubated with NAD over a course of time (Figure 1A). Additionally, when cADPR was added exogenously to parasite lysates, it was progressively hydrolysed over time, indicating that the parasite also possesses a cADPR hydrolase activity (Figure 1B). T. gondii cADPR cyclase activity showed an optimum pH of approx. 7.2 (Figure 1C) and was significantly inhibited by incubation with nicotinamide and dithiothreitol and was stimulated by Zn (an effect blocked by EDTA) (Figure 1D). These results indicate that the ADP-ribosyl cyclase in T. gondii has features similar to the well-characterized mammalian ADP-ribosyl cyclase CD38 [30–32]. As this assay is highly sensitive for cADPR, these results indicate the presence of cyclase and hydrolase enzymes that control the production and turnover of cADPR in T. gondii. Extraction of intracellular nucleotides in T. gondii revealed the presence of cADPR at the level of 0.6±0.02 pmol/mg of protein (mean±S.D., n=3) using the cyclin assay for detecting cADPR. Collectively, these findings indicate that cADPR is an endogenous nucleotide in T. gondii.
Figure 1. Synthesis and degradation of cADPR by T. gondii.
(A) Activity of ADP-ribosyl cyclase from T. gondii homogenates incubated with 1 mM NAD as described in the Experimental section. (B) Hydrolysis of cADPR. T. gondii homogenates were incubated with 10 μM cADPR and the remaining cADPR was determined as described in the Experimental section. (C) Extracts from T. gondii were assayed for cyclase activity using 0.2 mM NGD as a substrate, and effects of several inhibitors and stimulators of the cyclase activity and the optimal pH were tested. (D) Extracts were incubated with cyclase stimulators or inhibitors 1 min before the addition of the cyclase substrate NGD. The incubations were performed with: none (CTL), 1 mM nicotinamide (NM), 1 mM dithiothreitol (DTT), 1 mM ZnCl2 and 1 mM ZnCl2+1 mM EDTA. *P<0.05 indicates significant difference from control (Student's t test).
Calcium release by cADPR
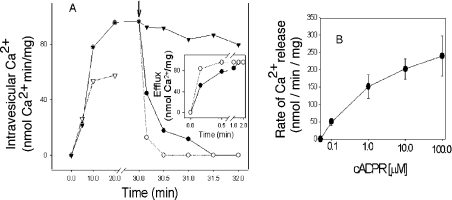
cADPR has been shown to stimulate calcium release in a variety of systems including sea-urchin eggs [36], T-lymphocytes [12] and neurosecretory cells [11]. Therefore we sought evidence that cADPR could effect the release of calcium from membranes of T. gondii in vitro. Microsomes prepared from tachyzoites of T. gondii demonstrated a time-dependent uptake of 45Ca2+ that was partially inhibited by thapsigargin (Figure 2A). This result is consistent with previous reports that thapsigargin causes the release of intracellular calcium in T. gondii [7]. Addition of IP3 or cADPR caused rapid release of calcium from T. gondii microsome vesicles (Figure 2A). Efflux was most rapid in the first 1 min and, then, the rate of release reached a plateau (Figure 2A, inset). Release of calcium from T. gondii microsomes showed a dose-dependent rate of increase with increase in the concentration of cADPR (Figure 2B).
Figure 2. cADPR induced Ca2+ release in T. gondii microsomes.
(A) Ca2+ uptake by T. gondii microsomes in the absence (▼) or presence (▽) of 10 μM thapsigargin. After 30 min, Ca2+ release was monitored after adding control diluent (▼), 10 μM IP3 (○) or 10 μM cADPR (●). The inset demonstrates the rate of Ca2+ efflux induced by cADPR or IP3. (B) Ca2+ release induced by different concentrations of cADPR using the same procedure as that described in (A). Ca2+ uptake and release in T. gondii microsomes were measured as described in the Experimental section.
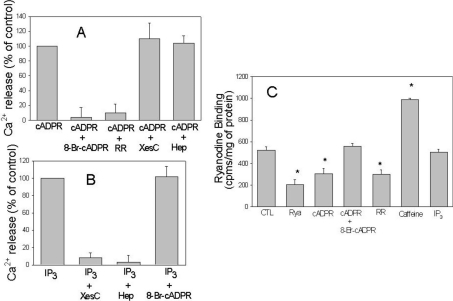
cADPR is known to cause the release of calcium from RyR through a process that is sensitive to several inhibitors [37]. The ability of cADPR to cause the release of calcium from T. gondii microsomes was efficiently blocked by 8-Br-cADPR and Ruthenium Red (Figure 3A), consistent with the properties of this inhibitor in blocking the activity of RyR in other systems [37]. In contrast, addition of xestospongin C or heparin, competitive inhibitors of IP3 channels [38], had no effect on the release of calcium by cADPR from T. gondii microsomes (Figure 3A). As shown in Figures 2 and 3, T. gondii microsomes also responded to IP3 and this response was not inhibited by Ruthenium Red or 8-Br-cADPR, but was potently inhibited by xestospongin C and heparin (Figure 3B). Consistent with these results, the binding of radiolabelled ryanodine to T. gondii microsomes was competitively inhibited by unlabelled ryanodine, cADPR and ruthenium, and stimulated by caffeine but not influenced by IP3 (Figure 3C). Collectively, these results indicate that T. gondii microsomes contain cADPR-responsive channels that release calcium and conform to the properties of RyRs characterized in other systems. Additionally, T. gondii microsomes appear to contain independent calcium-release channels that are stimulated by IP3.
Figure 3. cADPR and IP3 induced Ca2+ release in T. gondii microsomes.
(A) Ca2+ release was induced by 10 μM cADPR in the presence of different inhibitors including 100 μM 8-Br-cADPR, 100 μM Ruthenium Red (RR), 100 μM xestospongin C (XesC) or 1 mg/ml heparin (Hep). (B) Ca2+ release was induced by 10 μM IP3 in the presence or absence of inhibitors as described in (A). In (A, B), Ca2+ release was monitored using the same procedure as that described in Figure 2. (C) Binding of [3H]ryanodine to T. gondii microsomes was measured as described in the Experimental section. Microsomes were incubated for 10 min with 100 μM unlabelled ryanodine (Rya), 10 μM cADPR, 10 μM cADPR with 100 μM 8-Br-cADPR, 100 μM Ruthenium Red (RR), 10 mM caffeine and 10 μM IP3.
Role of cADPR in the secretion and motility of T. gondii
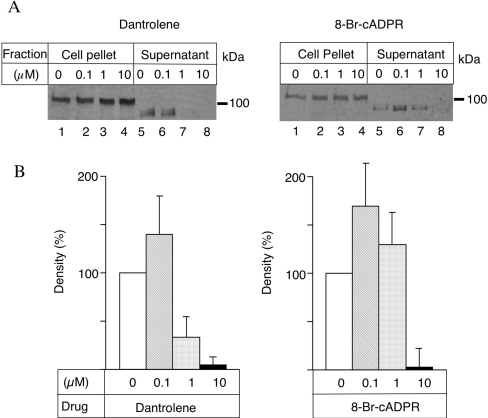
Increases in intracellular calcium stimulate microneme secretion in T. gondii and this step is essential for motility and cell invasion [6,7]. Previous studies have demonstrated a role for IP3 in this process, but the potential contribution of RyR has not been tested [27]. To evaluate the role of RyR in this process, we employed the non-hydrolysable analogue 8-Br-cADPR, which is a cell-permeant antagonist of the action of cADPR [39]. We also tested the effects of dantrolene, an inhibitor of RyR activities [37]. We examined the release of MIC2, a prominent protein in the micronemes that is implicated in cell binding and invasion [40]. Exocytosis of MIC2 is followed by C-terminal proteolytic processing that releases the protein from the cell surface; consequently, the secreted form migrates faster in SDS/PAGE [41]. Treatment of extracellular parasites with increasing doses of 8-Br-cADPR or dantrolene blocked parasite secretion of MIC2 into the supernatant (Figure 4A). This effect required preincubation and was most effective in parasites that were treated for 6 or 12 min before the induction of secretion (results not shown). Quantitative analysis indicated that the inhibition was significant at doses of ≤10 μM as shown by PhosphoImager analysis (Figure 4B).
Figure 4. Effect of cADPR antagonists on the secretion of MIC2.
Secretion of MIC2 into the supernatant after treatment with inhibitors. (A) Parasites were treated with dantrolene (left panel) or 8-Br-cADPR (right panel) or DMSO alone (both panels) on ice for 15 min. The parasites were further incubated for 5 min at 37 °C to induce secretion, separated by centrifugation and the supernatants were subjected to Western blotting. The form of the protein found in the supernatant migrates faster due to proteolytic processing at the C-terminus [41]. (B) Densities of the bands from secreted samples were quantified by a PhosphoImager as described in the Experimental section. The value 0 of the concentration represents secretion in DMSO that was set to 100%. Results shown in (B) are the means±S.D. for three experiments.
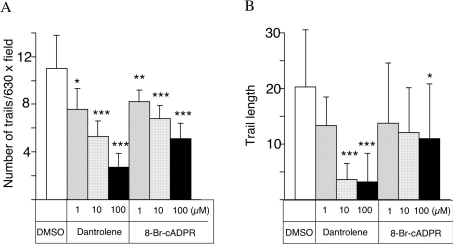
Oscillations in intracellular calcium have been shown to be important for modulating the gliding motility by T. gondii [8]. Therefore we tested whether treatment with inhibitors of the cADPR pathway would block the motility of the parasite. Gliding motility leads to characteristic trails being deposited on the substrate and these trails can be visualized by staining with antibodies to cell-surface proteins [8]. Treatment with dantrolene or 8-Br-cADPR led to a marked inhibition in the number of trails deposited on the substrate (Figure 5A) and a shortening of the average length of trails (Figure 5B). A significant inhibition of the average number of trails was observed even with the lowest concentrations of inhibitors (1 μM), indicating that this assay is more sensitive than the secretion of MIC2 shown in Figure 4. Although fewer parasites responded in the presence of inhibitors, a decrease in the average length of trails was only evident at higher levels of the inhibitors (10 and 100 μM for dantrolene and 8-Br-cADPR respectively) (Figures 5B and 5C).
Figure 5. Effects of cADPR antagonists on the gliding motility of T. gondii.
The formation and elongation of trails formed by the parasite during gliding motility was monitored by fluorescence microscopic observation. After treatment at 4 °C, gliding was induced by warming to 37 °C and trails were revealed by staining for the surface protein SAG1 using DG52 monoclonal antibody (see the Experimental section). The average number of trails and length of trails are indicated in (A) and (B) respectively. Results shown are the means±S.D. for three experiments. *P<0.05, **P<0.01, ***P<0.001 represent statistically significant differences determined by Student's t test.
Video analysis of the gliding motility
Since 8-Br-cADPR and dantrolene decreased microneme secretion and lowered the motility in static assays, we next examined parasites in real time to determine if RyR antagonists decreased the gliding. The treated parasites underwent three stereotypical behaviours: twirling, circular gliding and helical gliding as described previously [8]. The relative speeds of these movements in the treated cells were similar to controls, indicating that the basic gliding behaviour and velocity were not affected (results not shown). However, 8-Br-cADPR and dantrolene decreased the duration of time that the parasites spend gliding from an average of 35.0 s to 18.6 and 19.7 s respectively, and also decreased the number of parasites moving from 24% to 10.5 and 11% respectively (Table 1; P<0.05). Combined with the results from static assays described above, these results indicate that, by decreasing the amount of time that the parasites spend gliding, RyR antagonists diminished the net motility of T. gondii.
Table 1. Effects of treatment with RyR antagonists on gliding by T. gondii.
*P<0.05, significantly less than control (Student's t test).
| Mean % of parasites gliding | Average time for which gliding continues (s) | |
|---|---|---|
| Control | 24.0±0.81 | 35.0±2.4 |
| 1 μM 8-Br-cADPR | 10.5±0.86* | 18.6±2.7* |
| 1 μM Dantrolene | 11.0±1.66* | 19.7±2.7* |
Decreased motility was correlated with less intense intracellular calcium fluxes
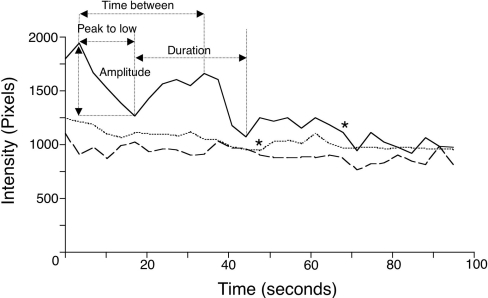
To determine how intracellular calcium levels were affected by 8-Br-cADPR and dantrolene treatment, we used parasites loaded with fluo-4 to visualize changes in calcium. Calcium fluxes correlate with gliding motility and often precede initiation of gliding, whereas calcium levels are quickly dampened in invading parasites [26]. We found that control gliding parasites underwent cycles of oscillating fluorescence that gradually decreased in intensity, whereas non-motile parasites did not undergo changes in fluorescence, as reported previously [8,26]. After treatment with 8-Br-cADPR or dantrolene, gliding parasites displayed less frequent and lower-intensity calcium oscillations relative to controls (Table 2). Plotting the kinetic changes in fluo-4 fluorescence in a representative parasite for each condition also demonstrated that parasites treated with RyR antagonists had slower, less significant fluxes in intracellular calcium (Figure 6).
Table 2. Duration of calcium transients in gliding control, 8-Br-cADPR and dantrolene-treated parasites.
Results are means±S.E.M. (s); n=9 (three parasites were analysed in each of the three separate experiments). Duration is the average time between the start of successive cycles; Peak-to-low is the time from the highest to the lowest intensity during a cycle; Time between is the average time between successive intensity peaks; Average amplitude is the average change in pixel intensity for the first flux, as shown in Figure 6. *P<0.05, significantly different from control (two-tailed Student's t test).
| Category | Duration | Peak-to-low | Time between | Average amplitude |
|---|---|---|---|---|
| DMSO | 22.3±2.4 | 15.1±0.6 | 24.6±0.8 | 390.0±40.5 |
| 1 μM 8-Br-cADPR | 41.5±5.4* | 26.6±5.5 | 46.7±9.2* | 197.7±38.7* |
| 1 μM Dantrolene | 39.2±5.0* | 28.8±6.0 | 43.4±6.5* | 234.5±22.2* |
Figure 6. Real-time calcium measurements in gliding parasites.
Plot of the absolute frame-by-frame fluorescence pixel intensity of one representative parasite per condition, showing fluo-4 fluorescence intensity oscillations during DMSO (——), 8-Br-cADPR (----) or dantrolene (····)-treated gliding. Gliding parasites were observed by fluorescence and phase microscopy, recorded at 1.5 s intervals, and analysed for fluorescence intensity during each frame. DMSO- or dantrolene-treated parasites stopped gliding movements during observation (indicated by an asterisk). Terms used in Table 2 (duration, peak-to-low, time between and intensity) are indicated.
DISCUSSION
The present study demonstrates that the protozoan parasite T. gondii has all the essential components of a cADPR-mediated signalling pathway that controls the release of intracellular calcium. This pathway governs protein secretion in an analogous manner to regulated secretion in higher eukaryotes. Exocytosis of parasite proteins from the micronemes is responsible for the release of adhesive proteins necessary for motility and cellular invasion. The presence of an RyR pathway in protozoa reflects the ancient ancestry of this signalling pathway and suggests that simple eukaryotes may provide useful models for the study of signalling pathways common to animals and plants.
Our studies provide evidence for a functional cADPR and ryanodine-responsive, calcium-release channel in T. gondii on the basis of several independent experiments. Treatment of intact parasites with caffeine or ryanodine causes increases in intracellular calcium as monitored by fura 2 [27]. Extracts of T. gondii are capable of synthesizing cADPR from NAD and to degrade it through hydrolase activity. The inhibition profile of T. gondii cADPR cyclase suggests that the T. gondii cyclase has pharmacological features similar to those of the well-characterized mammalian cyclase CD38 [30–32]. Furthermore, T. gondii microsomes that were loaded with 45Ca2+ in vitro released calcium in response to cADPR. This response was blocked by the antagonist 8-Br-cADPR and Ruthenium Red but not by xestospongin C, an inhibitor of IP3R. Finally, radiolabelled ryanodine bound specifically to T. gondii microsomes, indicating the presence of an RyR-type calcium-release channel in the parasite.
Intracellular calcium plays an important role in protein secretion and hence in motility and cell invasion by T. gondii. Agents that increase intracellular calcium levels trigger a rapid exocytosis of micronemes [6,7], organelles that contain adhesive proteins involved in substrate and host cell recognition [4]. Previous studies have shown that intracellular calcium stores in the parasite are required for motility and invasion [26]. These calcium storage pools are responsive to agonists of both IP3R- and RyR-type channels [27]. Results of our studies with in vitro-loaded microsomes from T. gondii demonstrate that these two channels release calcium in a mutually exclusive manner. We demonstrate in the present study that antagonists of RyR inhibit the secretion of microneme proteins and lead to decreased motility by the parasite. Since microneme protein secretion is essential for both motility and cell entry [6], these results imply that an RyR response pathway governs the calcium-mediated secretion associated with invasion. Previous studies have also shown that IP3-gated channels are important for parasite motility and invasion on the basis of inhibition with xestospongin C [27]. Collectively, these results indicate that both IP3 and RyR channels are important for efficient motility and cellular invasion, suggesting that they may work co-operatively, as in other systems [9]. At present, it is unclear what intracellular organelle houses the calcium that is released in response to IP3 or cADPR, although, based on other systems, a probable candidate is the ER. A less probable source is the acidocalcisome and the acidic calcium storage organelle found in protozoans, plants and prokaryotes [24,25]. Current knowledge about acidocalcisomes suggests that they are not involved in rapid calcium release and do not respond to agonists that stimulate intracellular calcium channels.
During gliding motility, cytoplasmic calcium levels in the parasite undergo significant oscillations, indicating that continued waves of release and re-uptake are involved in mediating gliding motility [26]. The precise relationship of these oscillations to microneme secretion is not clear; however, previous studies have shown that increases of intracellular calcium are both necessary and sufficient to stimulate secretion [6,7]. Although, in most systems, calmidazolium is a potent calmodulin inhibitor, we have previously shown that it acts as a calcium agonist in T. gondii [8]. Treatment with calmidazolium increases the frequency of oscillations and results in sustained secretion and motility [8]. One potential mechanism for this effect would be by enhancing the sensitivity of calcium channels to respond to natural stimuli, perhaps by allowing the influx of low levels of calcium that are known to stimulate release in other systems [9]. In the present study, we extend this correlation by showing that RyR antagonists dantrolene and 8-Br-cADPR decrease the amplitude and duration of calcium oscillations, thus indicating that calcium release is responsible for continued motility. Oscillations in cytoplasmic calcium in T. gondii probably involve re-uptake as well, a process typically mediated by the SERCA (sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase). Recent evidence indicates that the Chinese herbal medicine artemisinin targets the SERCA in Plasmodium falciparum and is the basis of selective antiparasitic action of this compound [42]. Identification of additional calcium response pathways in a parasitic protozoan is thus relevant to the identification of new targets for therapeutic intervention.
Three different RyR genes have been identified in mammalian cells and are often designated skeletal muscle, cardiac and brain isoforms [37]. Lower animals such as Caenorhabditis elegans and Drosophila typically contain only one IP3R-like channel and one RyR-like channel [43]. RyR and IP3 receptors in animals share similar domains and are thought to have evolved from a common ancestry [43]. Hydra [16], sponges [17] and plants [18] also respond to cADPR by releasing intracellular calcium. Despite the presence of a common signalling intermediate in cADPR, genes encoding RyR calcium-release channels have not been characterized from these organisms and plants do not contain IP3R or RyR genes homologous with those found in animals [44]. Consequently, calcium channels may have evolved independently in the early branching taxa that gave rise to metazoans. Protozoan parasites such as T. gondii branched very early in evolution either near or before the plant–animal–fungal kingdom split [45]. Apicomplexans also contain a vestigial plastid-like organelle that is a remnant of an algal symbiont [46]. Consequently, many genes in apicomplexans are more similar to plants than to animals, either owing to their early evolutionary branch point or to horizontal acquisition [47].
The presence of the RyR response pathway in T. gondii predicts that they should also contain genes encoding such channels. BLASTX and tBLASTN analysis of the recently completed 10× genome sequence of T. gondii (http://toxodb.org/ToxoDB.shtml) does not reveal any good candidate for either IP3 or RyR receptor on the basis of similarity to animal receptor genes (results not shown). BASTP comparison using the Arabidopsis SPRY domain-containing protein (NCBI accession no. NP_973963) identified two putative genes containing SPRY domains, one corresponding to the predicted open reading frame TwinScan_2519 and the other to TwinScan_0829. The SPRY domain is named so because it is a domain found in animal RyR genes and in SPL1a, a kinase that is involved in spore differentiation in Dictyostelium discoideum [48]. TwinScan_2519 encodes a putative protein of only 428 amino acids, which does not contain any significant hydrophobic transmembrane domain. TwinScan_0829 encodes a putative protein that contains a DEAD helicase domain and is probably involved in transcription regulation rather than calcium homoeostasis. Consequently, it is unlikely that either of these SPRY-containing genes in T. gondii encodes a functional RyR calcium-release channel.
Identification of calcium-release channels in protozoa will probably require further functional characterization before identification of the genes that encode them. Their identification might provide useful targets for intervention, given the essentialness of secretion for parasite motility and invasion of host cells. They may also provide fundamental knowledge about the origin and function of calcium-release channels and this would be beneficial for understanding these important pathways in other systems.
Acknowledgments
We thank S. Moreno (University of Georgia, Athens, GA, U.S.A.) and T. Steinberg (The George Washington University, Washington, DC, U.S.A.) for helpful comments and J. Suetterlin, K. Tang and M. Thomson for expert technical assistance. This work was partially supported by NIH (National Institutes of Health) grant AI34306 (to L.D.S.) and grants from the Mayo Foundation and Foundation of Anesthesia Research (to E.N.C.). K.N. was partially supported by the Uehara Memorial Foundation. L.D.S. is a Burroughs Wellcome Scholar in Molecular Parasitology.
References
- 1.Sibley L. D. Invasion strategies of intracellular parasites. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- 2.Dobrowolski J. M., Sibley L. D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell (Cambridge, Mass.) 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 3.Meissner M., Schluter D., Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- 4.Soldati D., Dubremetz J. F., Lebrun M. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int. J. Parasitol. 2001;31:1293–1302. doi: 10.1016/s0020-7519(01)00257-0. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers V. B., Giddings O. K., Sibley L. D. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1999;1:225–236. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers V. B., Sibley L. D. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 1999;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- 7.Carruthers V. B., Moreno S. N. J., Sibley L. D. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem. J. 1999;342:379–386. [PMC free article] [PubMed] [Google Scholar]
- 8.Wetzel D. M., Chen L. A., Ruiz F. A., Moreno S. N. J., Sibley L. D. Calcium-mediated protein secretion potentiates motility by Toxoplasma gondii. J. Cell Sci. 2004;117:5739–5748. doi: 10.1242/jcs.01495. [DOI] [PubMed] [Google Scholar]
- 9.Berridge M. J., Lipp P., Bootman M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 10.Prasad G. S., McRee D. E., Stura E. A., Levitt D. G., Lee H. C., Stout C. D. Crystal structure of Aplysia ADP ribosyl cyclase, a homologue of the bifunctional ectoenzyme CD38. Nat. Struct. Biol. 1996;3:957–964. doi: 10.1038/nsb1196-957. [DOI] [PubMed] [Google Scholar]
- 11.Guse A. H. Cyclic ADP-ribose: a novel Ca2+-mobilising second messenger. Cell Signalling. 1999;11:309–316. doi: 10.1016/s0898-6568(99)00004-2. [DOI] [PubMed] [Google Scholar]
- 12.Guse A. H., da Silva C. P., Berg I., Skapenko A. L., Weber K., Heyer P., Hohenegger M., Ashamu G. A., Schulze-Koops H., Potter B. V. L., et al. Regulation of calcium signaling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature (London) 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 13.Bennett D. L., Cheek T. R., Berridge M. J., De Semdt H., Parys J. B., Missiaen L., Bootman M. D. Expression and function of ryanodine receptors in nonexcitable cells. J. Biol. Chem. 1996;271:6356–6362. doi: 10.1074/jbc.271.11.6356. [DOI] [PubMed] [Google Scholar]
- 14.Hasan G., Rosbash M. Drosophila homologs of two mammalian intracellular Ca2+ release channels: identification and expression patterns of the inositol 1,4,5-triphosphate and the ryanodine receptor genes. Dev. Biol. 1992;116:967–975. doi: 10.1242/dev.116.4.967. [DOI] [PubMed] [Google Scholar]
- 15.Shiwa M., Murayama T., Ogawa Y. Molecular cloning and characterization of ryanodine receptor from fertilized sea urchin eggs. Am. J. Physiol. 2002;282:R727–R737. doi: 10.1152/ajpregu.00519.2001. [DOI] [PubMed] [Google Scholar]
- 16.Puce S., Basile G., Bavestrello G., Bruzzone S., Cerrano C., Giovine M., Arillo A., Zocchi E. Abscisic acid signaling through cyclic ADP-ribose in hydroid regeneration. J. Biol. Chem. 2004;279:39783–39788. doi: 10.1074/jbc.M405348200. [DOI] [PubMed] [Google Scholar]
- 17.Zocchi E., Carpeneto A., Bavestrello G., Giovine M., Bruzzone S., Guida L., Franco L., Usai C. The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid, and cyclic ADP ribose. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14859–14864. doi: 10.1073/pnas.261448698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y., Kuzma J., Marechal E., Graeff R., Lee H. C., Foster R., Chua N. H. Abscisic acid signaling through cyclic ADP ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- 19.Billker O., Tewari R., Franke-Fayard B., Brinkman V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell (Cambridge, Mass.) 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 20.Arrizabalaga G., Ruiz F. A., Morena S., Boothroyd J. C. Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. J. Cell Biol. 2004;165:653–662. doi: 10.1083/jcb.200309097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahoo N., Labruyere E., Bhattacharya S., Sen P., Guillen N., Bhattacharya A. Calcium binding protein 1 of the protozoan parasite Entamoeba histolytica interacts with actin and is involved in cytoskeletal dynamics. J. Cell Sci. 2004;117:3625–3634. doi: 10.1242/jcs.01198. [DOI] [PubMed] [Google Scholar]
- 22.Luo S., Vieira M., Graves J., Zhong L., Moreno S. N. A plasma membrane-type Ca2+-ATPase co-localizes with a vacuolar H+-pyrophosphatase to acidocalcisomes of Toxoplasma gondii. EMBO J. 2001;20:55–64. doi: 10.1093/emboj/20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo S., Rohloff P., Cox J., Yuyemura S. A., Docampo R. Trypanosoma brucei membrane-type Ca2+-ATPase 1 (TbPMC1) genes encode functional Ca2+-ATPases localized to the acidocalcisomes and plasma mebrane, and essential for calcium homeostasis and growth. J. Biol. Chem. 2004;279:14427–14439. doi: 10.1074/jbc.M309978200. [DOI] [PubMed] [Google Scholar]
- 24.Moreno S. N. J., Docampo R. Calcium regulation in protozoan parasites. Curr. Opin. Microbiol. 2003;6:359–364. doi: 10.1016/s1369-5274(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 25.Moreno S. N. J., Zhong L. Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem. J. 1996;313:655–659. doi: 10.1042/bj3130655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovett J. L., Sibley L. D. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J. Cell Sci. 2003;116:3009–3016. doi: 10.1242/jcs.00596. [DOI] [PubMed] [Google Scholar]
- 27.Lovett J. L., Marchesini N., Moreno S. N., Sibley L. D. Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from IP3/ryanodine sensitive stores. J. Biol. Chem. 2002;277:25870–25876. doi: 10.1074/jbc.M202553200. [DOI] [PubMed] [Google Scholar]
- 28.Klauke N., Plattner H. Caffeine-induced Ca2+ transients and exocytosis in Paramecium cells. A correlated Ca2+ imaging and quenched-flow/freeze-fracture analysis. J. Membr. Biol. 1998;161:65–81. doi: 10.1007/s002329900315. [DOI] [PubMed] [Google Scholar]
- 29.Kissmehl R., Huber S., Kottwitz B., Hauser K., Plattner H. Subplasmalemmal Ca-stores in Paramecium tetraurelia. Identification and characterisation of a sarco(endo)plasmic reticulum-like Ca2+-ATPase by phosphoenzyme intermediate formation and its inhibition by caffeine. Cell Calcium. 1998;24:193–203. doi: 10.1016/s0143-4160(98)90128-2. [DOI] [PubMed] [Google Scholar]
- 30.Beers K. W., Chini E. N., Lee H. C., Dousa T. P. Metabolism of cyclic ADP-ribose in opossum kidney renal epithelial cells. Am. J. Physiol. 1995;268:741–746. doi: 10.1152/ajpcell.1995.268.3.C741. [DOI] [PubMed] [Google Scholar]
- 31.Chini E. N., deToledo F. G., Thompson M. A., Dousa T. P. Effect of estrogen upon cyclic ADP-ribose metabolism:beta-estradiol stimulates ADP ribosyl cyclase in rat uterus. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5872–5876. doi: 10.1073/pnas.94.11.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zielinska W., Barata H., Chini E. N. Metabolism of cyclic-ADP-ribose: zinc is an endogenous modulator of the cyclase/NAD glycohydrolase ratio of a CD38-like enzyme from human seminal plasma. Life Sci. 2004;74:1781–1790. doi: 10.1016/j.lfs.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Graeff R., Lee H. C. A novel cycling assay for cellular cADP-ribose with nanomolar sensitivity. Biochem. J. 2001;361:379–384. doi: 10.1042/bj3610379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barata H., Thompson M. A., Zielinska W., Han Y. S., Mantilla C. B., Prakash Y. S. The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology. 2004;145:881–889. doi: 10.1210/en.2003-0774. [DOI] [PubMed] [Google Scholar]
- 35.Yusufi A. N. K., Cheng J., Thompson M. A., Dousa T. P., Warner G. M., Walker H. J., Grande J. P. cADP-ribose/ryanodine channel Ca2+ release signal transduction pathway in mesangial cells. Am. J. Physiol. 2001;281:91–102. doi: 10.1152/ajprenal.2001.281.1.F91. [DOI] [PubMed] [Google Scholar]
- 36.Galione A., White A., Willmott N., Turner M., Potter B. V. L., Watson S. P. cGMP mobilizes intracellular Ca2+ in sea urchin eggs by stimulating cyclic ADP-ribose synthesis. Nature (London) 1993;365:456–459. doi: 10.1038/365456a0. [DOI] [PubMed] [Google Scholar]
- 37.Coronado R., Morrissette J., Sukhareva M., Vaughan D. M. Structure and function of ryanodine receptors. Am. J. Physiol. 1994;266:C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- 38.Gafni J., Munsch J. A., Lam T. H., Catlin M. C., Costa L. G., Molinski T. F., Pessah I. N. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 39.Sethi J. K., Empson R. M., Bailey V. C., Potter B. V. L., Galione A. 7-Deaza-8-bromo-cyclic ADP-ribose, the first membrane-permeant, hydrolysis-resistant cyclic ADP-ribose antagonist. J. Biol. Chem. 1997;272:16358–16363. doi: 10.1074/jbc.272.26.16358. [DOI] [PubMed] [Google Scholar]
- 40.Huynh M. H., Barenau K. E., Harper J. M., Beatty W. L., Sibley L. D., Carruthers V. B. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 2003;22:2082–2090. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carruthers V. B., Sherman G. D., Sibley L. D. The toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 2000;275:14346–14353. doi: 10.1074/jbc.275.19.14346. [DOI] [PubMed] [Google Scholar]
- 42.Eckstein-Ludwig U., Webb R. J., van Goethem I. D. A., East J. M., Lee A. G., Kimura M., O'Neill P. M., Bray P. G., Ward S. A., Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature (London) 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 43.Sorrentino V., Barone V., Rossi D. Intracellular Ca(2+) release channels in evolution. Curr. Opin. Genet. Dev. 2000;10:662–667. doi: 10.1016/s0959-437x(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 44.Nagata T., Iizumi S., Satoh K., Ooka H., Kawai J., Carninci P., Hayashizaki Y., Otomo Y., Murakami K., Matsubara K., et al. Comparative analysis of plant and animal calcium signal transduction element using plant full-length cDNA data. Mol. Biol. Evol. 2004;21:1855–1870. doi: 10.1093/molbev/msh197. [DOI] [PubMed] [Google Scholar]
- 45.Baldauf S. L., Roger A. J., Wenk-Siefert I., Doolittle W. F. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science. 2000;290:972–977. doi: 10.1126/science.290.5493.972. [DOI] [PubMed] [Google Scholar]
- 46.Maréchal E., Cesbron-Delauw M. F. The apicoplast: a new member of the plastid family. Trends Plant Sci. 2001;6:200–205. doi: 10.1016/s1360-1385(01)01921-5. [DOI] [PubMed] [Google Scholar]
- 47.Huang J., Mullapudi N., Sicheritz-Ponten T., Kissinger J. C. A first glimpse into the pattern and scale of gene transfer in the Apicomplexa. Intl. J. Parasitol. 2004;34:265–274. doi: 10.1016/j.ijpara.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Ponting C., Schultz J., Bork P. SPRY domains in ryanodine receptors (Ca2+ release channels) Trends Biochem. Sci. 1997;22:193–194. doi: 10.1016/s0968-0004(97)01049-9. [DOI] [PubMed] [Google Scholar]