Abstract
Electrodes functionalised with weak electroactive microorganisms offer a viable alternative to conventional chemical sensors for detecting priority pollutants in bioremediation processes. Biofilm-based biosensors have been proposed for this purpose. However, biofilm formation and maturation require 24–48 h, and the microstructure and coverage of the electrode surface cannot be controlled, leading to poorly reproducible signal and sensitivity. Alternatively, semiconductive biocompatible coatings can be used for viable cell immobilization, achieving reproducible coverage and resulting in a stable biosensor response. In this work, we use a polydopamine (PDA)-based coating to immobilize Saccharomyces cerevisiae yeast viable cells on carbon screen printed electrodes (SPE) for Cu(II) detection, with potassium ferricyanide (K3[Fe (CN)6]) as a redox mediator. Under these conditions, the current output correlates with Cu (II) concentration, reaching a limit of detection of 2.2 µM, as calculated from the chronoamperometric response. The bioelectrochemical results are supported by standard viability assays, microscopy, and electrochemical impedance spectroscopy. The PDA coatings can be functionalised with different mutant strains, thus expanding the toolbox for biosensor design in bioremediation.
Keywords: Polydopamine, Biosensors, Bioremediation, Saccharomyces cerevisiae, Extracellular electron transfer
Subject terms: Electrocatalysis, Applied microbiology
Introduction
Since Leland Clark Jr developed the amperometric glucose electrode in 1962, electrochemical biosensors have found extensive use in clinical, environmental, industrial, and agricultural applications1. These biosensors integrate bioreporters (such as viable cells, aptamers, DNA, peptides, antigens) with electrochemical transducers (electrodes or field-effect transducers), making them suitable for miniaturization, batch fabrication, and integration with electronic systems. Moreover, the signal collection devices are cost-effective, portable, and energy-efficient, which is crucial for point-of-care diagnostics2–4. Despite these advantages, few electrochemical biosensors have reached the industrial stage, mainly due to the lack of affinity recognition between bioreporters and analytes. In addition to bacteria, unicellular yeasts have gained attention as bioreporters due to their robustness and ease of modification5–8. S. cerevisiae is particularly promising as it is tolerant to extreme environments, genetically tractable, and non-pathogenic9. S. cerevisiae can utilize both fermentable and non-fermentable carbon sources for energy production10, and it can also produce electrical signals while oxidizing organic compounds9,11,12. However, the low efficiency of extracellular electron transfer (EET) and the cumbersome process of cell immobilization still limit its practical applications13,14. Recently, the encapsulation of viable cells using PDA has been proposed to protect electroactive biofilms from acid shock15. PDA, a biopolymer derived from mussel adhesive proteins16, undergoes self-polymerization in alkaline conditions, forming strong covalent bonds with cell wall proteins, facilitating EET, and improving biocompatibility and stability15–19. Yeast cells in PDA coatings show potential for various applications, including biocatalytic asymmetric reduction and cytoprotection20,21. S. cerevisiae yeast cells, which display metabolic inhibition in the presence of metal pollutants, can be immobilized in PDA coatings, providing a viable option for electrochemical biosensing of Cu(II) and other metals in bioremediation22–25.
Currently, there is limited information on the bioelectrochemical response of yeast cells immobilized in PDA20,26. In this work, S. cerevisiae cells were immobilized on disposable SPE. Microscopy and electrochemical characterization of the coatings were conducted using different carbon sources, non-inhibitory Cu(II) concentration, and several respiratory mutants in the presence of K3[Fe (CN)6] as a redox mediator to enhance EET rate. PDA forms a homogeneous coating around yeast cells, enabling efficient EET. The high current output in the presence of the K3[Fe (CN)6] show promises for the development of stable Cu(II) biosensors for monitoring bioremediation processes.
Materials and methods
SPE setup and materials
SPEs (Ref. TE100, Zensor, Taiwan) with 3 mm diameter carbon working electrode (WE), carbon counter electrode (CE), silver (Ag) pseudo-reference electrodes (RE), along with a multichannel potentiostat (Multipalmsens4, Palmsens, The Netherlands) were used for all bioelectrochemical experiments. Unless otherwise stated, all electrochemical potentials are reported against the Ag pseudo-reference electrode. Cell immobilization was achieved using dopamine hydrochloride (CAS: 62-31-7, Purity (HPLC): ≥ 97.5%) (Aladdin, China). Electrochemical measurements were carried out in an electrolyte solution containing a carbon source (glucose (Glu), glycerol (Gly), or ethanol (Eth)), K3[Fe(CN)6], Magnesium dichloride (MgCl2), and MOPS (3-(N-morpholino)propanesulfonic acid) buffer (Aladdin, China).
Yeast strains and growth conditions
The S. cerevisiae strains used in this study were W303-1B (WT) (MATα ade2 leu2 his3 trp1 ura3), the retrograde response derivative ∆rtg2 (∆rtg2::LEU2) and the dysfunctional mitochondria derivative ∆hap4 (∆hap4:: KanMX4)27. All strains were maintained and sub-cultured on yeast extract peptone dextrose (YPD) agar and grown in liquid YPD medium containing 1% yeast extract (w/w) (Aladdin, China), 2% bactopeptone (w/w) (Gibco, Life Technologies, Waltham, MA, USA), and 2% glucose (w/w) (Sigma-Aldrich, St. Louis, MO, USA) at a shaking speed and temperature of 180 rpm and 30 ℃. In some experiments, glucose was substituted with glycerol or ethanol at the same concentration. A Varioskan-LUX microplate reader (Thermofisher Scientific, USA) was used to record yeast growth via absorbance measurement at 600 nm (OD600). Before immobilization in PDA coatings, yeast cells were centrifuged twice at 4000 g (Sorvall ST 8R, Thermofisher Scientific) and resuspended in 20 mM MOPS buffer, to remove extracellular debris and redox mediators that could affect the EET rate.
Assessment of S. cerevisiae tolerance to K3[Fe(CN)6]
The tolerance of S. cerevisiae to K3[Fe(CN)6] was determined by incubating the cells with various concentrations of the compound in 2 mL test tubes. First, the stock culture of each strain, maintained at 4 ℃, was aseptically transferred thrice to freshly prepared sterile YPD agar plate. Next, 5 mL of YPD medium was inoculated with a single colony from each strain and incubated overnight (16 h) at 30 ℃ and 180 rpm. The overnight cultures were then diluted with fresh YPD medium to a final OD600 of 0.05. K3[Fe(CN)6] stock solution was added to each tube containing YPD medium to obtain a final concentration ranging from 25 to 100 µM. Then, 800 µL of the culture was transferred into each well of a 48-well microtiter plate. Wells containing culture without K3[Fe(CN)6] and YPD medium only were used as controls and blanks, respectively. The cells were incubated in the microplate reader at 30 ℃ and 180 rpm for 48 h, with cell growth monitored by measuring OD600 every hour throughout the incubation.
Effects of Cu(II) on growth and viability of S. cerevisiae
The effect of Cu(II) on yeast cell growth and viability was assessed as follows: 5 mL YPD medium was inoculated with an axenic colony of each strain and incubated overnight (16 h) in 50 mL flasks at 30 ℃ and 180 rpm in a shaking incubator. The overnight cultures were diluted with fresh YPD medium to a final OD600 of 0.05 in flasks containing 50 mL sterile YPD medium supplemented with varying concentrations of CuSO4 (10–100 µM). Culture without CuSO4 and medium without cells were used as controls and blanks, respectively. The cultures were incubated 30 ℃ and 180 rpm for 48 h, and cell growth was monitored by measuring OD600 every hour throughout the incubation.
Cell viability was assessed by colony forming unit (CFU) counting on YPD agar plates. During incubation, 100 µL of aliquot was taken from each culture every 2 h until the end of the exponential growth phase (18 h). This was serially diluted in sterile milliQ water, inoculated onto YPD agar plates and incubated at 30 ℃ for 48 h. Colonies formed on each plate after incubation were counted, with the number of untreated colonies considered 100%, and the viability of treated colonies calculated as a percentage of untreated cells28.
Coatings preparation
For electrochemical analysis, S. cerevisiae cells and dopamine hydrochloride were used to prepare coatings following previously published protocols26. Briefly, cells were pre-inoculated overnight in YPD medium and then incubated in fresh YPD. The culture was centrifuged and resuspended in 20 mM MOPS buffer at pH 7 to enable self-polymerization of PDA with 50 mM glucose as a carbon source. This suspension was mixed with 10 mM dopamine hydrochloride solution in 1:1 ratio and magnetically stirred under aerobic conditions for 1 h to obtain a homogeneous solution. Following aerobic polymerization, 5 μL of the solution was drop-cast on the 3 mm WE surface of the SPE and left to dry at a controlled temperature of 26 ± 1 ℃ for 30 min. Electrochemical polymerization was performed with 20 repeated cyclic voltammetry (CV) scans between − 0.3 and + 0.5 V with a scan rate of 20 mV s−1. The number of cycles was optimised to avoid formation of oversized PDA coating (data not shown). PDA coatings were characterized using scanning electron microscopy (SEM), CV, chronoamperometry (CA), and EIS. Control experiments were performed using the same coatings preparation procedures with dead cells (cells were heat-killed by placing them in a hot water bath for 1 h at 100 ℃) and PDA-only coatings. All experiments were performed at fixed temperature of 26 ± 1℃ in 20 mL scintillation vials containing 18 mL electrolyte solution (20 mM MOPS buffer, pH 5 to avoid precipitation of copper hydroxide), 10 mM MgCl2, 50 mM glucose, and 50 µM K3[Fe(CN)6] under aerobic conditions. CuSO4 (0 to 100 µM) was added to selected experiments and the vials were magnetically stirred at 100 rpm for 120 s to ensure a homogeneous solution.
Field emission scanning electron microscope (FE-SEM)
The morphology of PDA coating was evaluated using a field emission scanning electron microscope (FE-SEM) (Sigma VP, ZEISS, Germany). Coatings were prepared as described above and cut to fit the size of stub/sample holder. Samples were fixed on the stub using conductive double-sided tape and gold-coated using an ion sputtering apparatus (SBC-12, KYKY, China) for 20–40 s at 5–10 mA. The samples were observed at a magnification of 1000 X with an area width of 3 to 10 mm under a vacuum pressure of 10 Pa. Other inspecting conditions such as signal collected (in-lens), conductor status (standard) and acceleration voltage (3 or 5 kV) were adjusted to obtain high-quality images.
Data analysis
All experiments were performed at least in triplicates. Separate cell cultures were prepared under the same conditions to obtain independent biological replicates. All electrochemical experiments were also performed using new SPE and sterilized electrochemical cells. For each dataset, the mean and standard deviation of experiments with three independent biological replicates (n = 3) are reported.
Results and discussion
Field emission scanning electron microscope (FE-SEM)
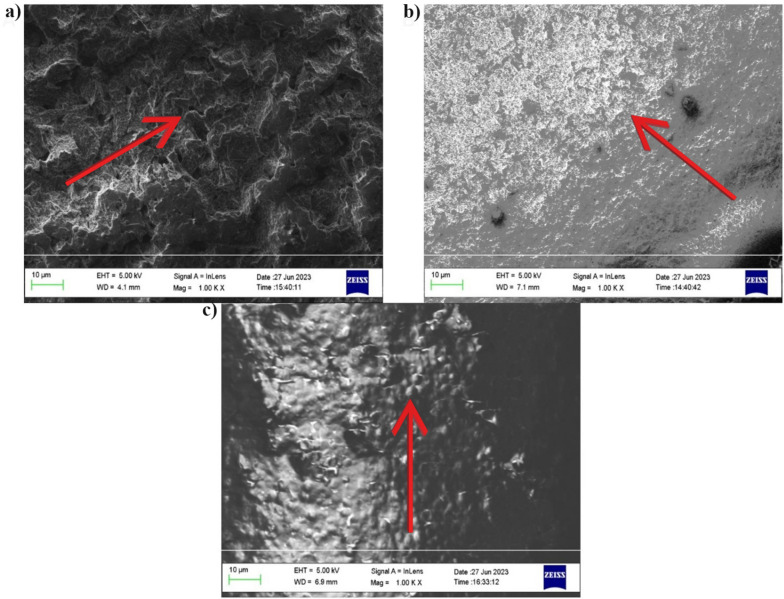
Figure 1a, b displays the bare and PDA-coated WE surface, respectively. The arrow in Fig. 1a indicates rough surface patches on the surface of SPE. The bare WE surface roughness favours coating adhesion and cell immobilization, which result in stable current output with time and better biosensor performance29. In Fig. 1b the arrow shows microstructures leading to formation of a smooth and uniform layer over the WE. The microstructures observed in the PDA coating may result from the self-polymerization of PDA and could contribute to enhancing cell adhesion and homogeneous distribution in the coating preparation.
Fig. 1.
(a) Bare surface of WE, (b) PDA-coated WE, (c) S. cerevisiae cells immobilized in PDA on the WE.
Figure 1c shows S. cerevisiae cells embedded within the PDA coating on the WE surface. The arrow displays cells that appear as round beads within the PDA matrix, indicating effective immobilization and homogeneous cell distribution, without the formation of cell clusters. The image is representative of the whole coated surface, as the uniform PDA coverage and consistent cell distribution are observed across all the WE tested.
Effects of K3[Fe(CN)6] on the growth of S. cerevisiae
The effect of the redox mediator K3[Fe(CN)6] on the growth of S. cerevisiae WT and mutant cells was determined using a multi-well plate assay. The results indicated that the growth rates were not significantly affected by the redox mediator at the concentrations tested (Figure S1). K3[Fe(CN)6] has been used both in microbial fuel cells (MFC) and electrochemical biosensors to increase EET rate from biofilms to electrodes30,31. For example, a S. cerevisiae MFC showed improved performance when 50 mM K3[Fe(CN)6] was added as external mediator30. Despite its mild toxicity, K3[Fe(CN)6] was successfully employed as redox mediator with fungi Candida albicans 32 at 100 μM concentration. With respect to another biocompatible redox mediator like 2-hydroxy-1,4-naphthoquinone (HNQ), K3[Fe(CN)6] is more stable and has a faster turnover rate33,34. Therefore, 50 µM K3[Fe(CN)6] was used in all electrochemical experiments carried out in this study.
Influence of Cu(II) on the growth and viability of S. cerevisiae
The ability to replicate and develop into colonies remains the gold standard for estimating microbial cell viability35. Thus, growth analysis and CFU counting of S. cerevisiae strains exposed to various Cu(II) concentrations were carried out according to standard CFU protocols. The results showed no significant difference in the growth rate of the three strains tested in the presence and absence of Cu(II) (Fig. 2 and Figure S2). Viability decreased with increasing concentrations of CuSO4 in each strain. However, the differences are not statistically significant (Tukey’s test, p < 0.05). Considering that ISO 10993-5:2009 standard define 30% viability decrease as the threshold for cytotoxicity36,37, we conclude that 100 µM CuSO4 is a suitable upper limit concentration for the proposed biosensor.
Fig. 2.

Viability of WT, ∆hap4 and ∆rtg2 S. cerevisiae strains incubated for 18 h in YPD medium supplemented with 10—100 µM CuSO4.
Depending on the concentration, Cu(II) can either play a key role in enzymatic metabolic processes or exert cytotoxic effects, causing cellular damage and loss of viability, especially during bioproduction processes such as fermentation24,38. In S. cerevisiae, increased level of the metalloenzyme superoxide dismutase (SOD) is directly linked to the presence of Cu(II) in the environment. Copper, as an inorganic cofactor, aids SOD in controlling reactive oxygen species levels, thereby protecting the cells from oxidative stress39. This is supported by increased SOD1 mRNA transcript levels in the presence of bathocuproine sulphonate, a Cu(II) chelator40. However, the level of viability maintained in the presence of Cu(II) varies widely among S. cerevisiae strains, depending on factors such as habitat of isolation and genotype. For example, YPD medium supplemented with 1 mM Cu(II) did not significantly affect the growth rate or viability of six different yeast strains comprising four S. cerevisiae and two Pichia pastoris41, whereas S. cerevisiae BH8 exposed to 0.5 mM CuSO4 showed enhanced growth in another study38. Consistent with our result, OD600 measurements showed no effect on the growth of S. cerevisiae strains S288c, AWRI1631, RM11, and YJM339 when exposed to 50 and 100 µM CuSO428. Fungi employ two primary metal detoxification mechanisms: surface binding (biosorption) or intracellular uptake during metabolism (bioaccumulation)42, and these traits vary widely among S. cerevisiae strains24. The low impact of Cu(II) on yeast viability in the 0–100 µM concentration range suggests its potential for electrochemical detection. Previous studies have developed biosensors using immobilized yeast cells for Cu(II). Genetically modified S. cerevisiae cells immobilized in alginate beads have been used to detect Cu(II) in the 1–100 μM range in drinking water and wastewater43. Our findings are consistent with previous studies, suggesting Cu(II) detection can be achieved electrochemically using viable S. cerevisiae cells immobilized in PDA coatings.
Electrochemical analysis
The effect of CuSO4 (0–100 µM) on the electroactivity of exponentially growing S. cerevisiae WT, Δhap4 and Δrtg2 cells was analyzed through PDA coatings using CV, CA and EIS.
Cyclic voltammetry
CV is a diagnostic technique for assessing the metabolic activity of attached cells44–46. Dead cells, PDA-only, and viable cells immobilized on the WE surface were characterized at 2 mV s−1 scan rate between − 0.30 and 0.52 V (Figure S3). After 14 h of growth, control experiments with PDA-only coating and dead yeast cells in PDA showed lower current density (j) than viable yeast cells in PDA. However, no distinct redox peaks were observed.
Electrochemical impedance spectroscopy
EIS was used to study response of four samples: viable S. cerevisiae WT cells immobilized in PDA with redox mediator (Sample 1), without redox mediator (Sample 2); PDA-only without redox mediator (Sample 3), and dead S. cerevisiae WT cells immobilized in PDA without redox mediator (Sample 4). Potentiostatic EIS was performed between − 100 and 400 mV with a step potential of 50 mV in the 50 kHz–0.05 Hz frequency range. EIS data were fitted with a two time-constant equivalent circuit using EC-Lab V11.61 (Bio-Logic, France). The Nyquist plot (Fig. 3a and Figure S7)) showed increasing real and imaginary components from Sample 1 to sample 4, indicating reduced impedance during EET in the presence of viable cells and redox mediator. For all samples impedance increase with the applied potential from − 100 mV to 400 mV. Depressed, incomplete semi-circles for each sample indicated non-ideal capacitance47. Figure 3b and Figure S8 shows the Bode phase plot, with two distinct features at f ~ 100 Hz and f ~ 1 Hz. The feature at f ~ 1 Hz represent charge transfer and double-layer capacitance on the coating-electrode interface, while the feature at f ~ 100 Hz describes charge accumulation on coating-electrolyte interface48.
Fig. 3.
(a) Nyquist plot; (b) Bode phase plot; (c) DRT plot of Sample 1 between −100 and 400 mV with a step potential of 50 mV; (d) Schematic of the equivalent circuit.
The phase angle never reached 90°, indicating non-ideal capacitive behavior, which can be modeled as a constant phase element (CPE)47, explaining the depressed semicircles observed in the Nyquist plot. The experimental EIS data was analyzed using the distribution function of relaxation times (DRT) and the corresponding time constants were calculated with an open-source Matlab-based DRT tool prior to modeling with equivalent circuit fitting (Fig. 3c and Figure S9)49. From DRT analysis, a leftward shift and reduction in time constants with increasing voltages for all samples was observed. This typically represents faster relaxation times, meaning that the electrochemical reactions or charge transfer processes involving the immobilized yeast cells are occurring more rapidly, indicating that the PDA layer provides a stable and conductive interface for the yeast cells50.
Given that the DRT analysis and phase angle revealed two distinct time constants, a Randles equivalent circuit comprising the resistance of electrolyte in series with two resistor-CPE circuits (Fig. 3d) was selected to represent the system51. In the equivalent circuit, represent the electrolyte resistance , and represent the CPE at the solution-coating interface, and represents the charge transfer resistance at the same interface. , and similarly model the non-ideal capacitance and charge transfer resistance at the coating-electrode interface51. The use of CPE accounts for the irregularities on the surface due to the roughness of the SPE and inhomogeneities in the coating52. Since CPE was employed in the model, the effective capacitance was calculated using the Brug Eq. (1). Brug’s equation account for both the electrolyte resistance and the behavior associated with surface distributions48,53,54.
| 1 |
where is the electrolyte resistance and , , and represent the CPE parameters for the coating-electrode interface.
The analysis of calculated from the equivalent circuit fitting showed variation across samples and with the applied potential (Figure S6), indicating that electrolyte diffusion in the coating depends on its composition and activity. Moreover, no significant reduction was observed in R1 in the presence of mediator (Figure S6). Figure 4 shows the for the coating-electrode interface calculated from the equivalent circuit fitting for all samples at different potentials. The interfacial resistance for sample 4 (dead cells) was higher than samples 2 and 1 (viable cells), while sample 3 had the lowest resistance. This effect suggests a facilitated charge-limited process and confirms the increased EET from the cells in sample 2 and 1. In contrast to interfacial resistance, effective capacitance showed an inverse trend, with sample 1 and 2 (viable cells) displaying higher capacitance in the fitted data compared to sample 4 (dead cells). This indicates the formation of a space charge layer in sample 1 and 2 (viable cells) due to higher EET, resulting in double-layer capacitance at electrode-biofilm interface. These results are consistent with our hypothesis and with the results obtained from CV and CA as well.
Fig. 4.
(a) Interfacial resistance for all samples. (b) Effective capacitance for all samples.
Chronoamperometry
In long-term experiments (e.g., 24–48 h), the current output is due to the growth and respiration of the electricigens55. For short-term experiments with coatings, where cell growth can be neglected, the current output can be interpreted as steady-state electrode respiration of the immobilized cells. Thus, short-term CA is a suitable method to determine the effects of environmental conditions, nutrient concentrations, genetic modifications, pollutants, etc. on the cell respiration rate. In an initial set of experiments, S. cerevisiae WT cells were grown in rich medium with glucose as fermentative carbon source and glycerol and ethanol as non-fermentative carbon sources. Unlike glucose, which is preferentially metabolized by yeast cells even in the presence of oxygen (glucose catabolite repression), glycerol and ethanol stimulate respiration. Results showed that the current output was significantly higher under respiratory metabolism compared to fermentative metabolism (Fig. 5).
Fig. 5.

CA of PDA coating with S. cerevisiae WT cells with glucose, glycerol, and ethanol as the carbon source at 0.4 V, with different concentration of CuSO4 (0, 10, 50, 100 µM) under aerobic conditions.
Interestingly, it was reported that S. cerevisiae respired aerobically in the mitochondria in the presence of glycerol as a carbon source, producing 30% more ATP than in a medium containing glucose as the carbon source56. Vasylkovska et al. (2015) also demonstrated that the medium supplemented with glycerol or ethanol as carbon sources lead to 6.9- and 3.3-fold increase in metabolic rates, respectively, compared to media containing glucose57. Although no significant difference was recorded in the SOD activity of the strains utilizing each of these carbon sources, catalase activity was found to be 3.3% higher in the glycerol medium relative to the glucose medium. Relative growth data under these conditions suggest that copper has a stimulatory effect in glycerol or ethanol medium (Figure S4 and S5) according to58. Beyond its positive effect on respiratory rate, copper can also ameliorate oxidative stress. As previously reported, trace amount of Cu(II) can increase SOD activity. This, combined with the high catalase activity in the presence of glycerol, could result in a more balanced redox state, leading to the increase in current output59.
The CA results were compared at 2500 s, where the current output reached a steady state for all samples, and the EET rate increase by about 30% for cells cultured in glycerol or ethanol at almost all concentrations of CuSO4, compared to glucose. However, at all concentrations of CuSO4, the current output decreased in all samples (Fig. 6 and Table S1), indicating that short-term exposure to CuSO4 causes a rapid change in extracellular respiration rate. Thus, PDA coatings can be used to prepare biosensors for the rapid detection of respiratory stress agents, such as toxic metals and other pollutants. The data obtained from the dose-dependent response of the coating (Fig. 6) was analysed for linear fitting, and the LOD was calculated according to the 3Sb/m criterium, where m is the slope of the linear range and Sb the standard deviation of the intercept 60. Interestingly, while glucose showed an LOD of 2.2 µM, the higher respiration rate with glycerol and ethanol as carbon sources resulted in lower LODs (1.8 and 1.4 µM, respectively), with high sensitivity for CuSO4 (Table S3).
Fig. 6.
Current density of PDA coating with S. cerevisiae WT cells with glucose, glycerol, and ethanol as the carbon source with different concentration of CuSO4 (0, 10, 50, 100 µM) under aerobic conditions after 2500 s of incubation at E = 0.4 V.
Having observed that respiratory metabolism might affect electrical cell response, CA was performed on two mutant strains, Δhap4 and Δrtg2, lacking the catalytic subunit of the hap2-5 transcriptional complex required for the control of the tricarboxylic acid (TCA) cycle and electron transport, and the upstream regulator of the mitochondrial retrograde pathway, respectively. The three strains were cultured in medium containing glucose as the sole carbon source. Both mutations improved the EET response by more than 80% without CuSO4. However, in the presence of CuSO4, the current output increase was approximately 30% for Δhap4 and become negligible for the Δrtg2 strain (Figs. 7, 8 and Table S2), indicating a perturbation in the cell’s metabolic state that could be associated with Cu (II) induced stress. These observations align with cell growth data, which show that copper can enhance cell growth in both mutants compared to WT (Figure S4). Conversely, the increased EET response in the presence of copper could be due to reduced oxidative stress in the mutants. Control experiments with PDA coatings containing heat-killed cells and PDA-only coatings showed negligible current output.
Fig. 7.
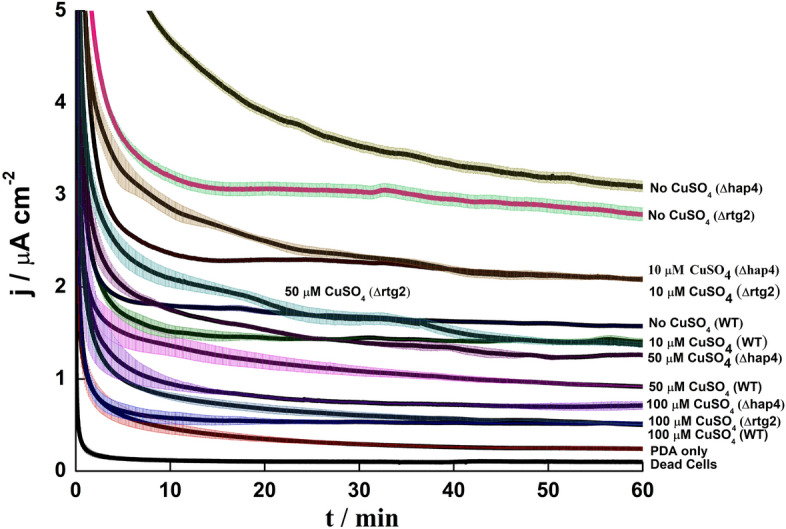
CA of Current density of PDA coating with S. cerevisiae WT, Δhap4 and Δrtg2 cells at 0.4 V with different concentration of CuSO4 (0, 10, 50, 100 µM) under aerobic conditions.
Fig. 8.
Current density of PDA coating with S. cerevisiae WT, Δhap4 and Δrtg2 cells at 0.4 V with different concentration of CuSO4 (0, 10, 50, 100 µM) under aerobic conditions after 2500 s of incubation.
The higher rate of respiration observed in Δhap4 and ∆rtg2 agrees with previous reports61 and could be due to the higher mitochondrial mass, especially in Δhap4, where growth appeared accelerated compared to WT cells in the absence of stress. Additionally, the higher copper resistance in in both mutant strains may be an effect of dysfunctional mitochondria43.
Overall, this confirms an interplay between rtg2 and hap4 in controlling mitochondrial function62. The higher respiration rate in Δhap4 and Δrtg2 strains grown on glucose as the carbon source translate into a lower LOD and higher sensitivity for CuSO4 (Table S4). Moreover, the results obtained in this work with biosensors prepared using metabolic and genetic manipulations immobilized on SPE showed improvement in all aspects such LoD, R2 and sensitivity in comparison to the results obtained by glassy carbon bioelectrode26. The current set of biosensors provided at least 10 percent higher in LoD, improved R2 (except ethanol in case of different carbon sources and Δhap4 in case of mutant) as well as a minimum of twofold increase in sensitivity26. Looking at the CA traces, either two or three phases can be observed (Figs. 5 and 7): (a) a rapid capacitive discharge in the initial 5 min; (b) a slow decrease of the current output, which lasts longer at low CuSO4 concentrations; and c) a plateau phase, during which the current remain stable over time. Virtually no current was observed for dead cells, indicating the biological origin of the current output. The current output for dead cells waseven lower than that of PDA only, likely due to the polyelectrolyte nature of PDA, which facilitate EET63.
The CuSO4 concentration of 100 µM is likely close to the minimum inhibitory concentration (MIC)64–66, meaning cells are slowly dying or being inhibited under these conditions. For practical applications, the concentration of the analyte should be kept lower than the MIC to avoid damaging the sensing element. The comparision between CFU counting and CA analysis indicates that both methods show similar trends. However, in the case of CFU counting, growth inhibition is minimal, with a slight decrease in percentage viability only observed after 18 h of exposure (Fig. 2). Thus, PDA coatings offer better sensitivity and more rapid detection compared to conventional growth-based assays.
Conclusions
This study focuses on creating a sustainable and economical biosensor by coating viable S. cerevisiae cells in polydopamine on a screen-printed electrode, to detect Cu(II), with potential applications in bioremediation. The immobilization process preserves the yeast cells’ metabolic functionality and stability, enabling effective electrochemical analysis over time through chronoamperometry. The use of disposable carbon screen-printed electrodes improves the sensor’s portability and its detection accuracy with respect to conventional carbon rod electrode for laboratory studies. Scanning electron microscopy and electrochemical impedance spectroscopy were applied to validate these enhancements, providing further information on the electron transfer process in the biocoating. Further study will employ genetically modified yeast cells to detect specific metal contaminants for environmental monitoring and bioremediation applications.
Supplementary Information
Acknowledgements
E.W. and O.O. were recipients of PhD fellowships from the Italian Ministry of University and Research: Piano Stralcio «Ricerca e innovazione 2015-2017» del Fondo per lo Sviluppo e la Coesione. Anno Accademico 2020/2021—Ciclo XXXVI” [“Avviso D.D. 1233/2020”] for the projects “Biosensors development for precision agriculture” to N.G. and “BioSense-Biosensors for IoT-based Precision Farming” to C.G. This work was partially supported by the Nottingham Ningbo China Beacons of Excellence Research and Innovation Institute [budget code I01220800007].
Author contributions
Conceptualization: Enrico Marsili, Nicoletta Guaragnella, Ehtisham Wahid; Methodology: Enrico Marsili, Nicoletta Guaragnella, Matteo Grattieri; Investigation: Ehtisham Wahid, Sunday Olakunle Oguntomi, Run Pan, Ohiemi Benjamin Ocheja; Data curation: Ehtisham Wahid, Enrico Marsili, Sunday Olakunle Oguntomi; Writing—original draft: Ehtisham Wahid, Sunday Olakunle Oguntomi, Enrico Marsili, Nicoletta Guaragnella; Writing—review & editing: all authors.
Data availability
The full dataset for this work can be requested via email to Enrico Marsili (enrico.marsili@nottingham.edu.cn).
Declarations
Competing interests
The authors have no conflict of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ehtisham Wahid and Ohiemi Benjamin Ocheja have contributed equally to this work
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86702-8.
References
- 1.Clark, L. C. & Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann N. Y. Acad. Sci.102, 29–45 (1962). [DOI] [PubMed] [Google Scholar]
- 2.Labib, M., Sargent, E. H. & Kelley, S. O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem Rev116(16), 9001–9090 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Wu, J. et al. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. TrAC Trends Anal. Chem.26, 679–688 (2007). [Google Scholar]
- 4.Minteer, S. D. Advances in electroanalytical chemistry. J Am Chem Soc140(8), 2701–2703 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Adeniran, A., Sherer, M. & Tyo, K. E. Yeast-based biosensors: design and applications. FEMS Yeast Res15(1), 1–15 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Jarque, S. et al. Yeast biosensors for detection of environmental pollutants: Current state and limitations. Trends Biotechnol34(5), 408–419 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Martin-Yken, H. Yeast-based biosensors: current applications and new developments. Biosensors (Basel) 10(5) (2020). [DOI] [PMC free article] [PubMed]
- 8.Roda, A. et al. Analytical strategies for improving the robustness and reproducibility of bioluminescent microbial bioreporters. Anal Bioanal Chem401(1), 201–211 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Lian, J., Mishra, S. & Zhao, H. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab Eng50, 85–108 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Turcotte, B. et al. Transcriptional regulation of nonfermentable carbon utilization in budding yeast. FEMS Yeast Res10(1), 2–13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavulu, S. et al. Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: Influence of redox condition and substrate load. Bioresour Technol102, 2751–2757 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Sayed, E. et al. Yeast extract as an effective and safe mediator for the Baker’s-Yeast-based microbial fuel cell. Ind. Eng. Chem. Res.54, 3116–3122 (2015). [Google Scholar]
- 13.Lovley, D. Electromicrobiology. Annu. Rev. Microbiol.66, 391–409 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Xie, X., Criddle, C., and Cui, Y. Design and fabrication of bioelectrodes for microbial bioelectrochemical systems. Energy Environ. Sci.8 (2015).
- 15.Holten-Andersen, N. & Waite, J. H. Mussel-designed protective coatings for compliant substrates. J Dent Res87(8), 701–709 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papov, V. et al. Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel mytilus edulis. J. Biol. Chem.270, 20183–20192 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Liu, Y., Ai, K., and Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields.Chem. Rev.114 (2014). [DOI] [PubMed]
- 18.Yang, S. H. et al. Mussel-inspired encapsulation and functionalization of individual yeast cells. J Am Chem Soc133(9), 2795–2797 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Fakhrullin, R. et al. Cyborg cells: Functionalisation of living cells with polymers and nanomaterials. Chem. Soc. Rev.41, 4189–4206 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Wang, L. et al. Polydopamine nanocoated whole-cell asymmetric biocatalysts. Chem Commun (Camb)53(49), 6617–6620 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Du, Q., et al. Protection of electroactive biofilm from extreme acid shock by polydopamine encapsulation. Environ. Sci. Technol. Lett. 4 (2017).
- 22.Nogueirol, R. C. et al. Sequential extraction and availability of copper in Cu fungicide-amended vineyard soils from Southern Brazil. J Hazard Mater181(1–3), 931–937 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Claus, H. Copper-containing oxidases: occurrence in soil microorganisms, properties, and applications, pp. 281–313 (2010).
- 24.Capece, A. et al. Yeast starter as a biotechnological tool for reducing copper content in wine. Front Microbiol8, 2632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García Esparza, M. et al. Copper content of grape and wine from Italian farms. Food Addit. Contaminants23, 274–280 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Benjamin Ocheja, O. et al. Polydopamine-immobilized yeast cells for portable electrochemical biosensors applied in environmental copper sensing. Bioelectrochemistry157, 108658 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Guaragnella, N. et al. Yeast growth in raffinose results in resistance to acetic-acid induced programmed cell death mostly due to the activation of the mitochondrial retrograde pathway. Biochim Biophys Acta1833(12), 2765–2774 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Rong-Mullins, X. et al. Proteomic and genetic analysis of the response of S. cerevisiae to soluble copper leads to improvement of the antimicrobial function of cellulosic copper nanoparticles. Metallomics9(9), 1304–1315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Police Patil, A. V., et al. Recent advances in electrochemical immunosensors with nanomaterial assistance for signal amplification.Biosensors (Basel). 13(1) (2023). [DOI] [PMC free article] [PubMed]
- 30.Gunawardena, A., Fernando, S. & To, F. Performance of a yeast-mediated biological fuel cell. Int J Mol Sci9(10), 1893–1907 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tharali, A., Sain, N. & Osborne, J. Microbial fuel cells in bioelectricity production. Front. Life Sci.9, 1–15 (2016). [Google Scholar]
- 32.Olaifa, K. et al. Electroanalysis of Candida albicans biofilms: A suitable real-time tool for antifungal testing. Electrochimica Acta389, 138757 (2021). [Google Scholar]
- 33.Wark, A. W. et al. Bioaffinity detection of pathogens on surfaces. J Ind Eng Chem16(2), 169–177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santoro, C., et al. Sub-toxic concentrations of volatile organic compounds inhibit extracellular respiration of Escherichia coli cells grown in anodic bioelectrochemical systems. Bioelectrochemistry. 112 (2016). [DOI] [PubMed]
- 35.Meyer, C. T. et al. A high-throughput and low-waste viability assay for microbes. Nat Microbiol8(12), 2304–2314 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa, E. et al. Spent yeast waste streams as a sustainable source of bioactive peptides for skin applications. Int. J. Mol. Sci.24, 2253 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vicensoto Moreira, M., et al. Ascorbic acid as a modulator of inflammatory response against Candida albicans. Future Microbiol. 19 (2024). [DOI] [PMC free article] [PubMed]
- 38.Sun, X. et al. Effect of high Cu2+ stress on fermentation performance and copper biosorption of Saccharomyces cerevisiae during wine fermentation. Food Sci. Technol.39 (2018).
- 39.Berterame, N. M. et al. Copper homeostasis as a target to improve Saccharomyces cerevisiae tolerance to oxidative stress. Metab Eng46, 43–50 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Montllor-Albalate, C. et al. Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae. Redox Biol21, 101064 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vulpe, C. et al. Copper accumulation efficiency in different recombinant microorganism strains available for bioremediation of heavy metal-polluted waters. Int. J. Mol. Sci.24, 7575 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tufail, M. et al. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ.850, 157961 (2022). [DOI] [PubMed] [Google Scholar]
- 43.Vopálenská, I., Váchová, L., and Palkova, Z. New biosensor for detection of copper ions in water based on immobilized genetically modified yeast cells. Biosens. Bioelectron.72 (2015). [DOI] [PubMed]
- 44.Marsili, E. et al. Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl Environ Microbiol74(23), 7329–7337 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babauta, J. et al. Electrochemically active biofilms: Facts and fiction. A review. Biofouling28(8), 789–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalenko, Y. et al. Electrochemistry of Escherichia coli JM109: Direct electron transfer and antibiotic resistance. Biosens. Bioelectron.32, 219–223 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Pires, L. et al. Online monitoring of biofilm growth and activity using a combined multi-channel impedimetric and amperometric sensor. Biosens Bioelectron47, 157–163 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Hirschorn, B. et al. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochimica Acta55, 6218–6227 (2010). [Google Scholar]
- 49.Wan, T., et al. Influence of the discretization methods on the distribution of relaxation times deconvolution: Implementing radial basis functions with DRTtools. Electrochimica Acta184 (2015).
- 50.Astorga, S. E., Hu, L. X., Marsili, E. & Huang, Y. Ordered micropillar array gold electrode increases electrochemical signature of early biofilm attachment. Mater. Des.185, 108256 (2020). [Google Scholar]
- 51.Astorga, S. et al. Electrochemical signature of Escherichia coli on Ni micropillar array electrode for early biofilm characterization. ChemElectroChem6, 4674–4680 (2019). [Google Scholar]
- 52.Salazar, P., Martín, M., and González-Mora, J. In situ electrodeposition of cholesterol oxidase-modified polydopamine thin film on nanostructured screen printed electrodes for free cholesterol determination. J. Electroanal. Chem.837 (2019).
- 53.Dominguez-Benetton, X. et al. The accurate use of impedance analysis for the study of microbial electrochemical systems. Chem. Soc. Rev.41, 7228–7246 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Samantha Michelle, G. et al. On the use of a constant phase element (CPE) in electrochemistry. Curr. Opinion Electrochem.36, 101133 (2022). [Google Scholar]
- 55.Sánchez, C. et al. Microbial electrochemical technologies: Electronic circuitry and characterization tools. Biosens Bioelectron150, 111884 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Maslanka, R., Zadrag-Tecza, R., and Kwolek-Mirek, M. Linkage between carbon metabolism, redox status and cellular physiology in the yeast. Genes (Basel)11(7) (2020). [DOI] [PMC free article] [PubMed]
- 57.Vasylkovska, R., Petriv, N. & Semchyshyn, H. Carbon sources for yeast growth as a precondition of hydrogen peroxide induced hormetic phenotype. Int. J. Microbiol.2015, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirchman, P. A. & Botta, G. Copper supplementation increases yeast life span under conditions requiring respiratory metabolism. Mech Ageing Dev128(2), 187–195 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang, Q. & Zhou, B. Copper and manganese induce yeast apoptosis via different pathways. Mol Biol Cell18(12), 4741–4749 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nunez-Bajo, E. et al. Electrogeneration of gold nanoparticles on porous-carbon paper-based electrodes and application to inorganic arsenic analysis in white wines by chronoamperometric stripping. Anal Chem89(12), 6415–6423 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Guaragnella, N., et al. Signaling sustains mitochondrial respiratory capacity in HOG1-dependent osmoadaptation. Microorganisms9(9) (2021). [DOI] [PMC free article] [PubMed]
- 62.Di Noia, M. A., et al. Inactivation of HAP4 accelerates RTG-dependent osmoadaptation in Saccharomyces cerevisiae. Int. J. Mol. Sci.24(6) (2023). [DOI] [PMC free article] [PubMed]
- 63.Buscemi, G. et al. Bio-inspired redox-adhesive polydopamine matrix for intact bacteria biohybrid photoanodes. ACS Appl Mater Interfaces14(23), 26631–26641 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohsumi, Y., Kitamoto, K. & Anraku, Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J Bacteriol170(6), 2676–2682 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azenha, M., Vasconcelos, M. T. & Moradas-Ferreira, P. The influence of Cu concentration on ethanolic fermentation by Saccharomyces cerevisiae. J Biosci Bioeng90(2), 163–167 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Mrvcić, J. et al. Optimization of bioprocess for production of copper-enriched biomass of industrially important microorganism Saccharomyces cerevisiae. J Biosci Bioeng103(4), 331–337 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset for this work can be requested via email to Enrico Marsili (enrico.marsili@nottingham.edu.cn).