Abstract
1. Smooth muscle cells from guinea-pig urinary bladder were studied at an extracellular Ca2+ concentration ([Ca2+]o) of 3.6 mM and 36 degrees C. Fluorescence of Indo-1 was used to monitor the cytosolic calcium concentration ([Ca2+]i) and its changes ([Ca2+]i transients) induced by step membrane depolarizations. 2. During a 6 s depolarization step from -60 to 0 mV [Ca2+]i increased from a resting 118 +/- 22 nM to 1150 +/- 336 nM and decayed to a sustained level of 295 +/- 62 nM. The experiments were designed to evaluate the contribution of the release of intracellularly stored Ca2+ to components of the depolarization-induced [Ca2+]i transient, i.e. 'phasic', which decayed during a maintained depolarization step, and 'tonic' which constituted the sustained elevation of [Ca2+]i above resting level. 3. A short (1 s) application of 10 mM caffeine mimicked the phasic component. After wash-out of caffeine, the subsequent depolarization induced a [Ca2+]i transient with reduced peak, the degree of suppression depending on the interval between wash-out of caffeine and depolarization. The phasic component of the depolarization and the caffeine-induced [Ca2+]i transients were not additive but saturative. 4. The phasic component was largely abolished in the continuous presence of 10 mM caffeine. It was also abolished by a 10 min cell dialysis of 10 microM ryanodine from the pipette solution and was strongly reduced by dialysis of 5 microM thapsigargin. Changes of the tonic component of the depolarization-induced [Ca2+]i transient were much less pronounced with all three interventions. 5. The tonic component of the depolarization-induced [Ca2+]i transient was increased when [Ca2+]o was elevated briefly before a depolarization close to 0 mV, whereas the phasic component was not significantly changed. Similarly, brief application of 1 microM Bay K 8644 increased the tonic component several-fold without modifying significantly the phasic component. 6. It is concluded that depolarization-induced influx of Ca2+ through L-type Ca2+ channels induces the release of Ca2+ from intracellular caffeine-sensitive stores which constitutes the major part of the phasic component. Ca2+ release superimposes on the effects of Ca2+ influx through L-type Ca2+ channels, the non-inactivating part of which constitutes the tonic component of the [Ca2+]i transient. Since the two processes interact, a dissection by simple subtraction is not possible.
Full text
PDF



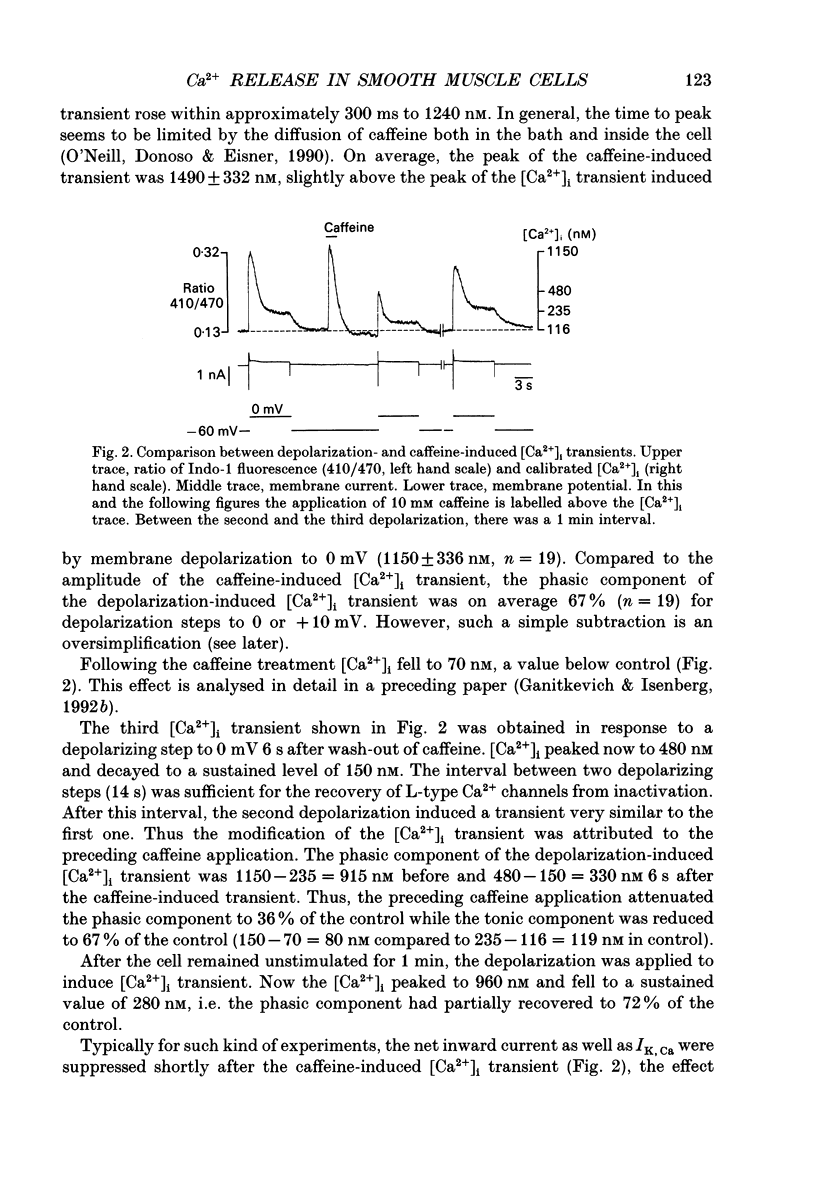




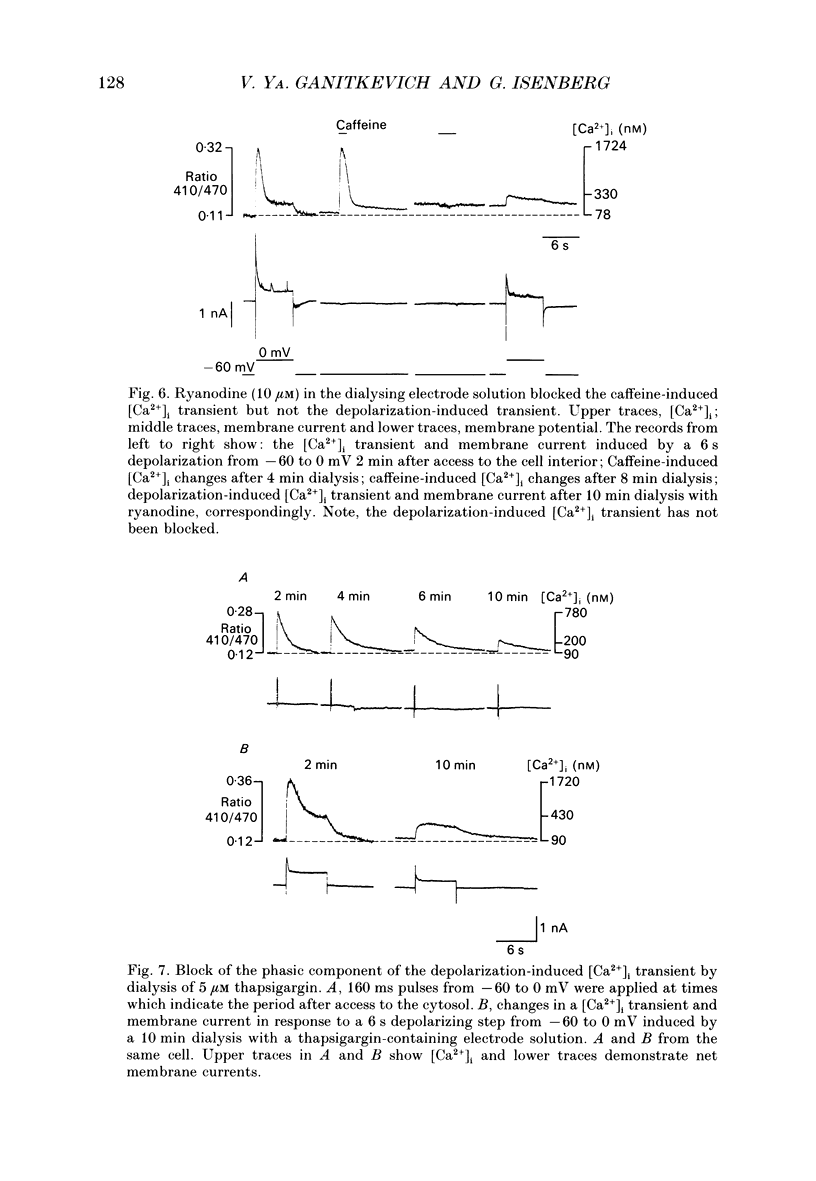







Selected References
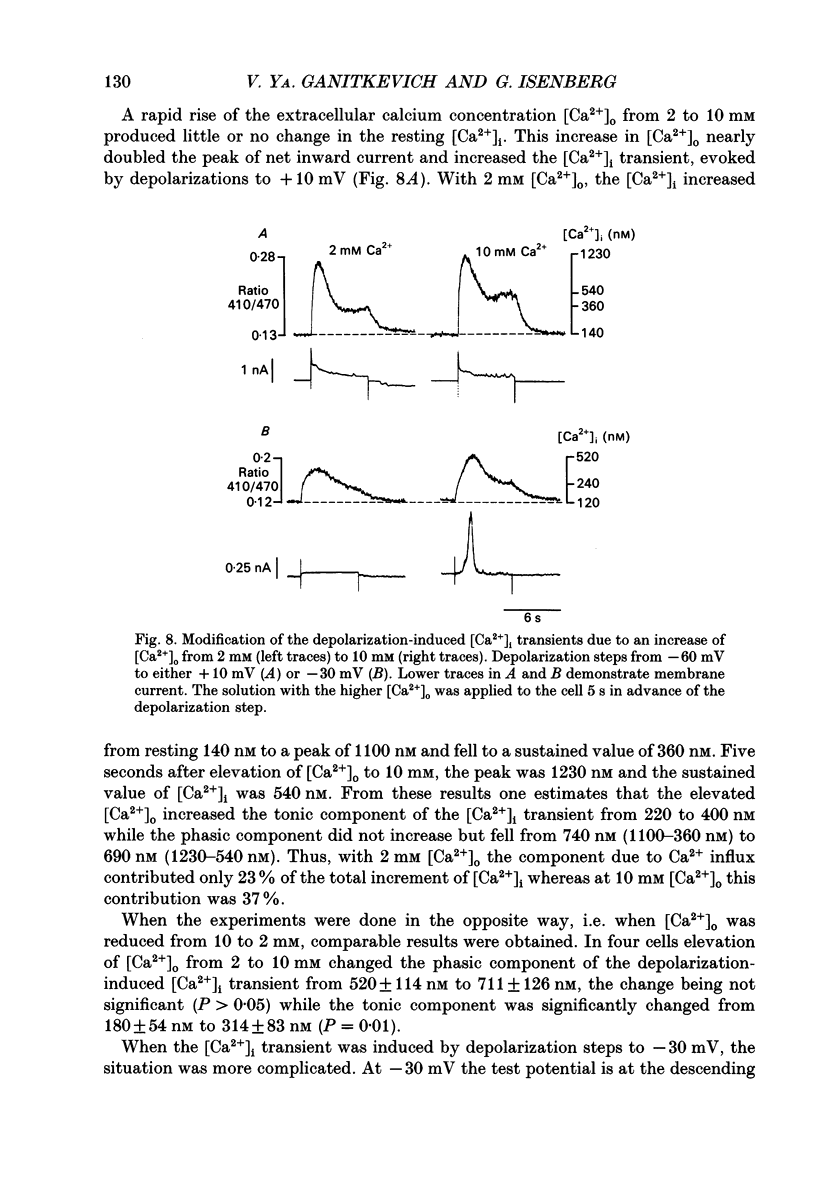
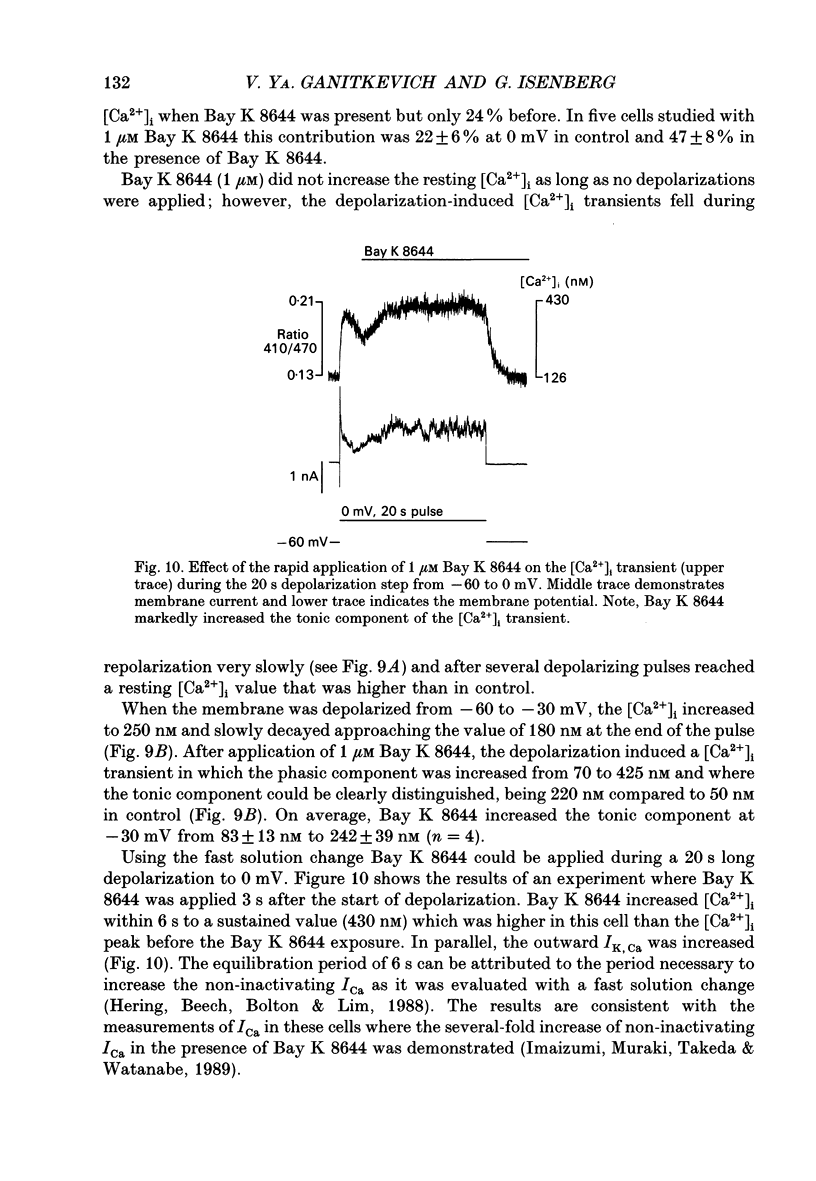
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J. H., Ghosh T. K., Wang J. C., Gill D. L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991 May 15;266(14):8801–8806. [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V Y. a., Isenberg G. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. J Physiol. 1991 Apr;435:187–205. doi: 10.1113/jphysiol.1991.sp018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Caffeine-induced release and reuptake of Ca2+ by Ca2+ stores in myocytes from guinea-pig urinary bladder. J Physiol. 1992 Dec;458:99–117. doi: 10.1113/jphysiol.1992.sp019408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987 Nov;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Saturation of calcium channels in single isolated smooth muscle cells of guinea-pig taenia caeci. J Physiol. 1988 May;399:419–436. doi: 10.1113/jphysiol.1988.sp017089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering S., Beech D. J., Bolton T. B., Lim S. P. Action of nifedipine or BAY K8644 is dependent on calcium channel state in single smooth muscle cells from rabbit ear artery. Pflugers Arch. 1988 May;411(5):590–592. doi: 10.1007/BF00582383. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A., Darling E., Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch. 1991 May;418(4):353–359. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I., Okamoto Y. Ryanodine reveals multiple contractile and relaxant mechanisms in vascular smooth muscle: simultaneous measurements of mechanical activity and of cytoplasmic free Ca2+ level with fura-2. Br J Pharmacol. 1990 Aug;100(4):677–684. doi: 10.1111/j.1476-5381.1990.tb14075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. D., Hering S., Bolton T. B. The action of caffeine on inward barium current through voltage-dependent calcium channels in single rabbit ear artery cells. Pflugers Arch. 1990 Jun;416(4):462–466. doi: 10.1007/BF00370755. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Calcium release mechanisms in smooth muscle. Jpn J Pharmacol. 1990 Dec;54(4):345–354. doi: 10.1254/jjp.54.345. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Takeda M., Watanabe M. Measurement and simulation of noninactivating Ca current in smooth muscle cells. Am J Physiol. 1989 Apr;256(4 Pt 1):C880–C885. doi: 10.1152/ajpcell.1989.256.4.C880. [DOI] [PubMed] [Google Scholar]
- Kanmura Y., Missiaen L., Raeymaekers L., Casteels R. Ryanodine reduces the amount of calcium in intracellular stores of smooth-muscle cells of the rabbit ear artery. Pflugers Arch. 1988 Dec;413(2):153–159. doi: 10.1007/BF00582525. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Inositol trisphosphate releases stored calcium to block voltage-dependent calcium channels in single smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):437–441. doi: 10.1007/BF00497770. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Takemura H., Obie J. F., Thastrup O., Putney J. W., Jr Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990 Jun;258(6 Pt 1):C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Wuytack F., Raeymaekers L., De Smedt H., Droogmans G., Declerck I., Casteels R. Ca2+ extrusion across plasma membrane and Ca2+ uptake by intracellular stores. Pharmacol Ther. 1991;50(2):191–232. doi: 10.1016/0163-7258(91)90014-d. [DOI] [PubMed] [Google Scholar]
- O'Neill S. C., Donoso P., Eisner D. A. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Bolton T. B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991 Sep;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am J Physiol. 1989 Feb;256(2 Pt 2):H328–H333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Schneider P., Hopp H. H., Isenberg G. Ca2+ influx through ATP-gated channels increments [Ca2+]i and inactivates ICa in myocytes from guinea-pig urinary bladder. J Physiol. 1991;440:479–496. doi: 10.1113/jphysiol.1991.sp018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990 Apr;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]


