Abstract
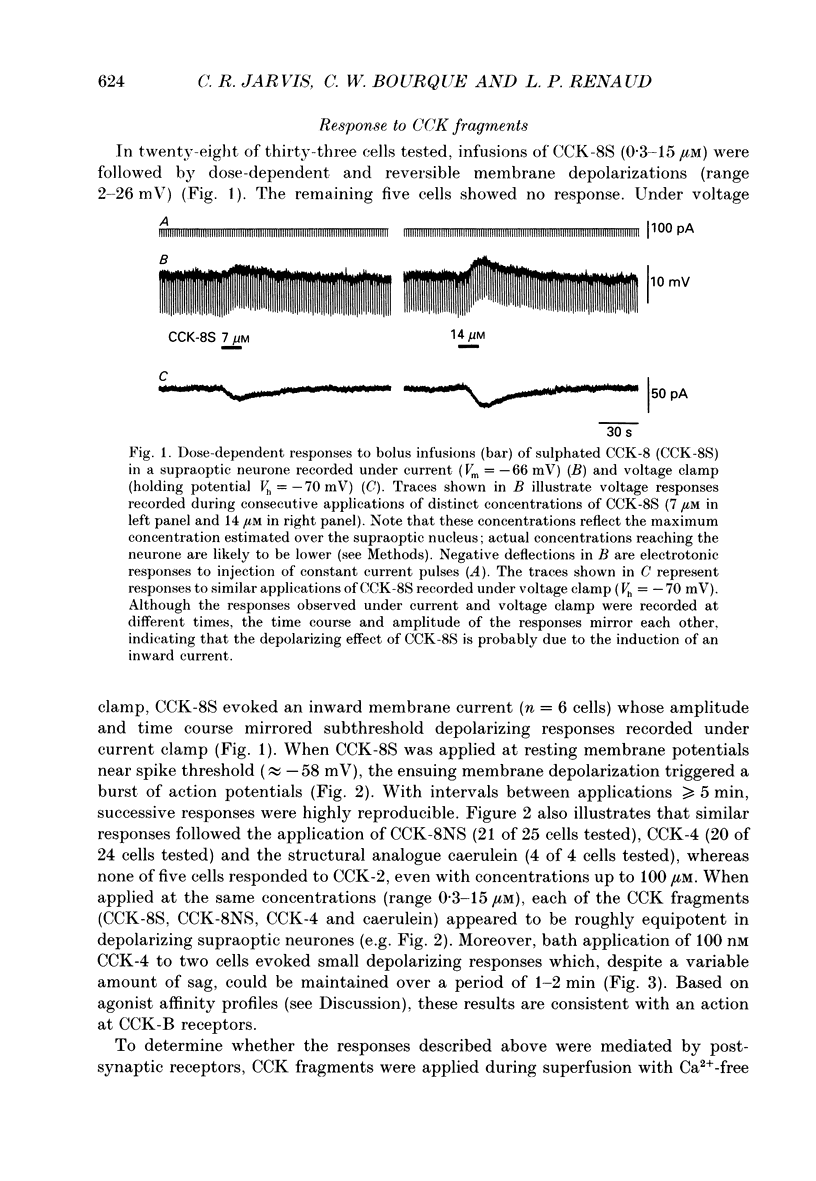
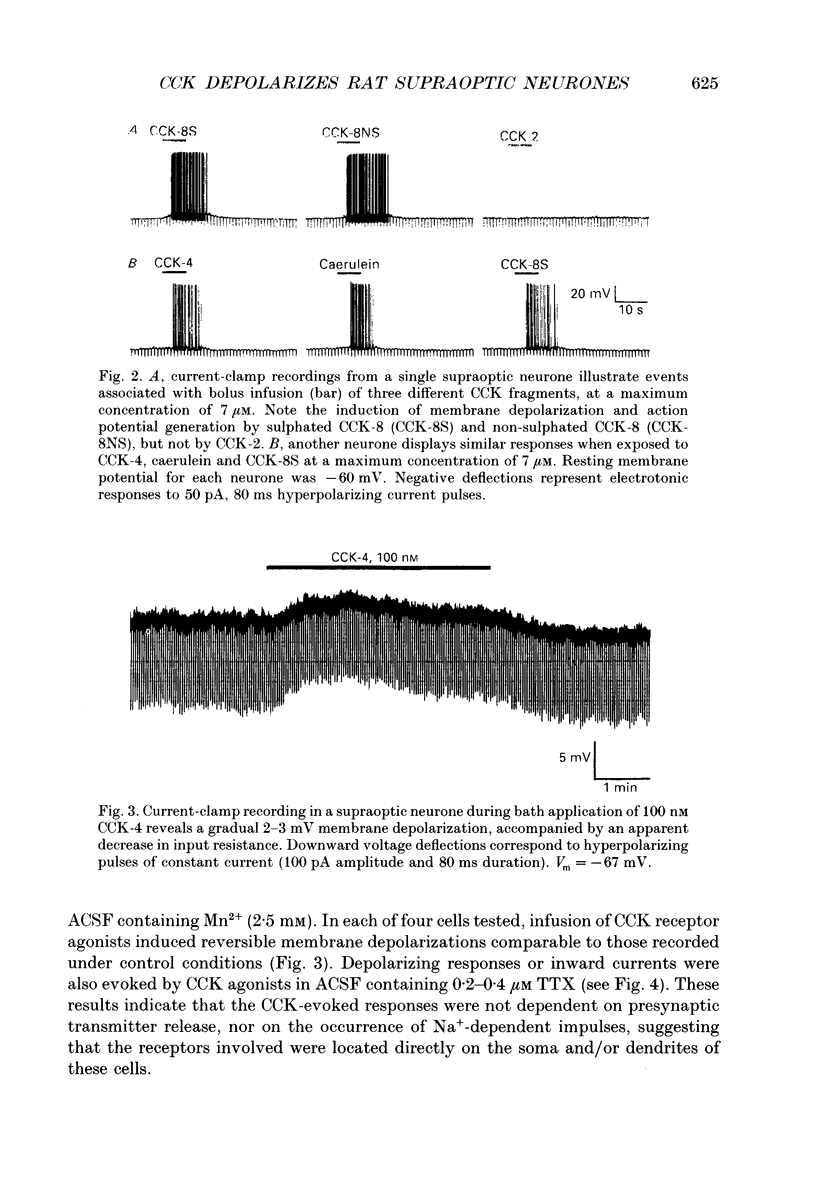
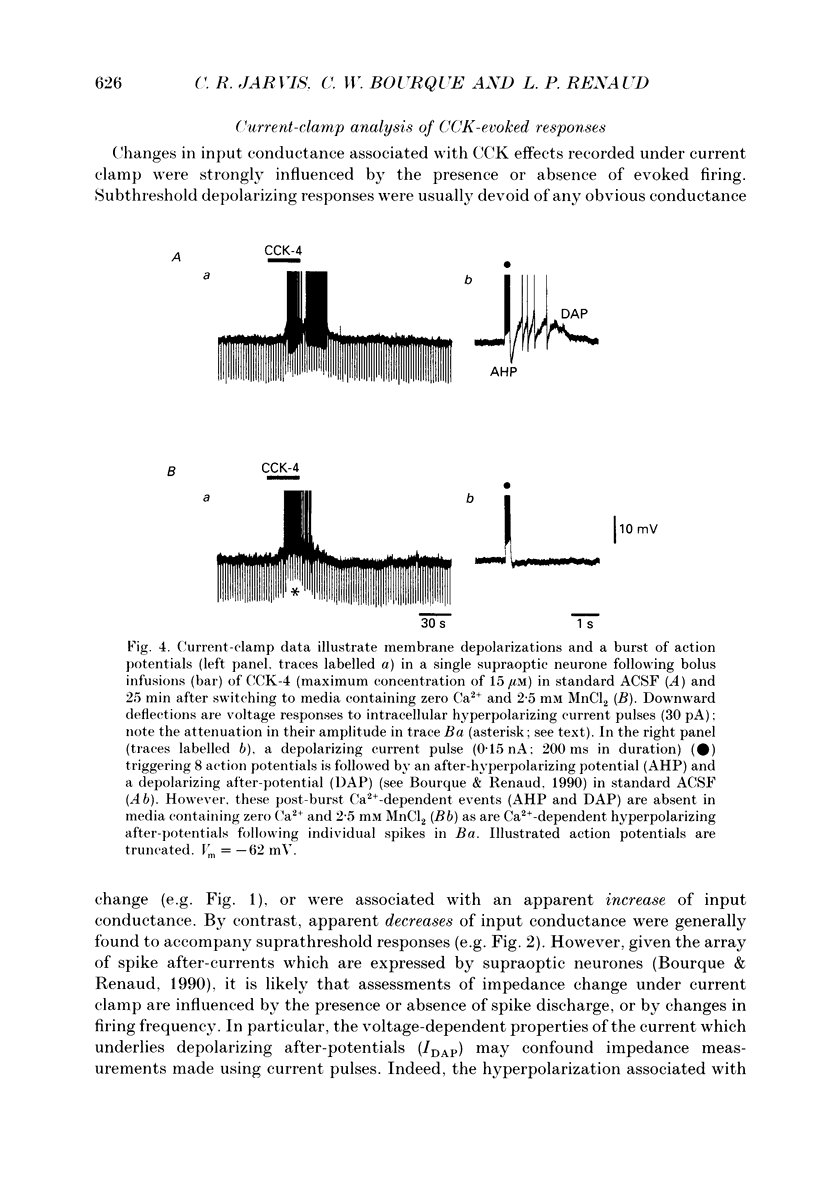
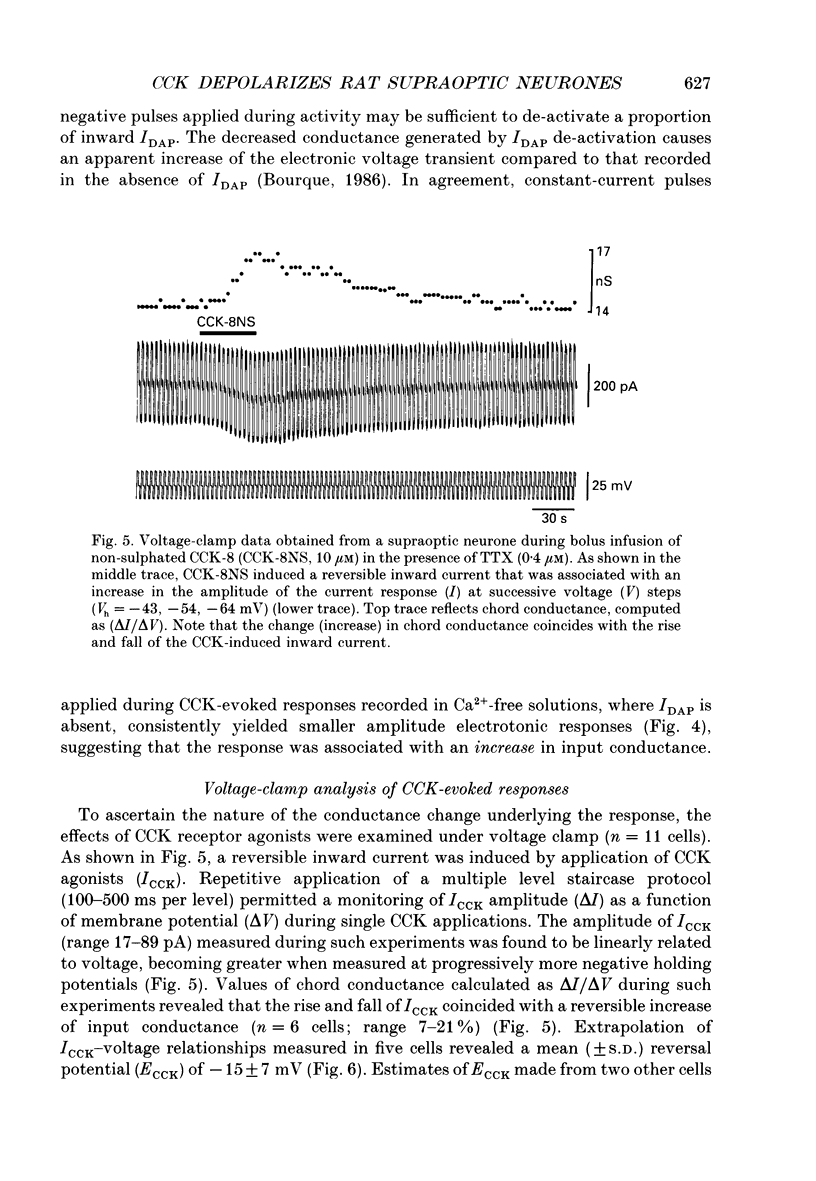
1. Cholecystokinin is co-localized within the oxytocin- and, to a lesser extent, vasopressin-synthesizing magnocellular neurones in the hypothalamic supraoptic and paraventricular nuclei. These nuclei are also prominent binding sites for cholecystokinin. In the present study we used intracellular current- and voltage-clamp recordings from fifty-seven supraoptic nucleus cells, maintained in superfused explants of rat hypothalamus, to assess their membrane responses to exogenous cholecystokinin and define the nature of their cholecystokinin receptors. 2. In a majority of the fifty-seven cells tested, bolus infusions into the superfusion media of cholecystokinin fragments (maximum concentrations estimated at 0.3-15 microM) were followed within 1-5 s by a transient and reversible membrane depolarization. Active peptides included sulphated cholecystokinin octapeptide (26-33) (28 of 33 cells responded), non-sulphated cholecystokinin octapeptide (26-33) (21 of 25 cells responded), cholecystokinin tetrapeptide (30-33) (20 of 24 cells responded and caerulein (4 of 4 cells responded). None of five cells responded to cholecystokinin (26-28). Depolarizing responses to cholecystokinin analogues persisted in the presence of tetrodotoxin (0.2-0.4 microM), and in Ca(2+)-free solutions containing MnCl2 (2.5 mM). 3. Under voltage clamp, cholecystokinin fragments evoked an inward current accompanied by an increase in membrane conductance. The amplitude of the inward current varied linearly as a function of membrane voltage, with an extrapolated reversal potential of approximately -15 mV. Reversal potentials were not altered by chloride injection. These features suggest that cholecystokinin activates a non-selective cationic conductance. 4. Active cholecystokinin analogues were approximately equipotent in their depolarizing actions, a feature that supports the activation of cholecystokinin-B type receptors. Moreover bath application of 200 nM L-365,260, an antagonist with a high affinity for cholecystokinin-B receptors, reversibly attenuated the cholecystokinin-induced responses in four of six cells tested. 5. These observations indicate that cholecystokinin can directly influence the excitability of rat supraoptic nucleus neurones and provide evidence for an additional site where this peptide may act within the hypothalamo-neurohypophysial axis.
Full text
PDF


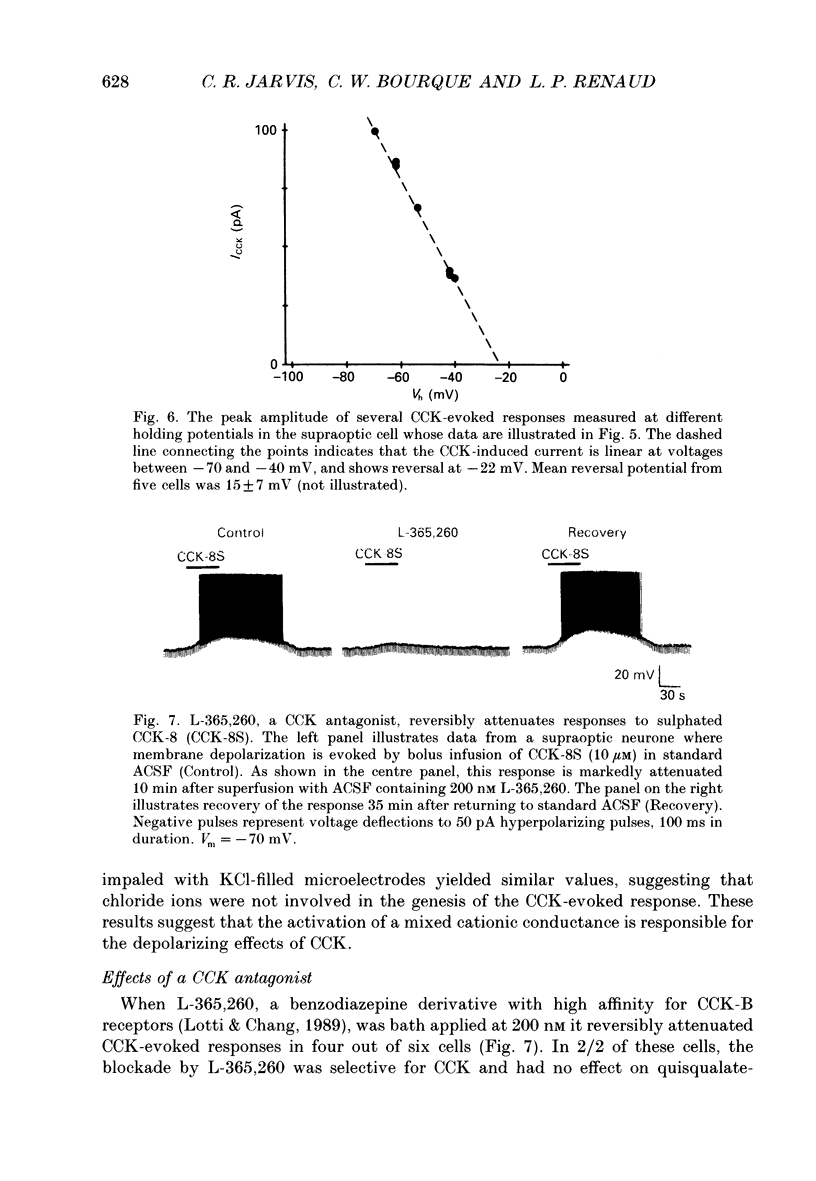




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akesson T. R., Micevych P. E. Binding of 125I-cholecystokinin-octapeptide in the paraventricular but not the supraoptic nucleus is increased by ovariectomy. Brain Res. 1986 Oct 15;385(1):165–168. doi: 10.1016/0006-8993(86)91560-x. [DOI] [PubMed] [Google Scholar]
- Boden P. R., Hill R. G. Effects of cholecystokinin and pentagastrin on rat hippocampal neurones maintained in vitro. Neuropeptides. 1988 Aug-Sep;12(2):95–103. doi: 10.1016/0143-4179(88)90037-6. [DOI] [PubMed] [Google Scholar]
- Boden P., Hill R. G. Effects of cholecystokinin and related peptides on neuronal activity in the ventromedial nucleus of the rat hypothalamus. Br J Pharmacol. 1988 May;94(1):246–252. doi: 10.1111/j.1476-5381.1988.tb11521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neurosci Lett. 1986 Oct 8;70(2):204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Cobbett P., Smithson K. G., Hatton G. I. Immunoreactivity to vasopressin- but not oxytocin-associated neurophysin antiserum in phasic neurons of rat hypothalamic paraventricular nucleus. Brain Res. 1986 Jan 1;362(1):7–16. doi: 10.1016/0006-8993(86)91392-2. [DOI] [PubMed] [Google Scholar]
- Crawley J. N. Clarification of the behavioral functions of peripheral and central cholecystokinin: two separate peptide pools. Peptides. 1985;6 (Suppl 2):129–136. doi: 10.1016/0196-9781(85)90145-7. [DOI] [PubMed] [Google Scholar]
- Day N. C., Hall M. D., Hughes J. Modulation of hypothalamic cholecystokinin receptor density with changes in magnocellular activity: a quantitative autoradiographic study. Neuroscience. 1989;29(2):371–383. doi: 10.1016/0306-4522(89)90064-x. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Cholecystokinins in rat cerebral cortex: identification, purification and characterization by immunochemical methods. Brain Res. 1980 Apr 21;188(1):155–165. doi: 10.1016/0006-8993(80)90564-8. [DOI] [PubMed] [Google Scholar]
- Gaudreau P., St-Pierre S., Pert C. B., Quirion R. Cholecystokinin receptors in mammalian brain. A comparative characterization and visualization. Ann N Y Acad Sci. 1985;448:198–219. doi: 10.1111/j.1749-6632.1985.tb29919.x. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Campbell N. J., Shaw T. M., Woodruff G. N. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987 Sep;7(9):2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Woodruff G. N. Differentiation of central cholecystokinin receptor binding sites using the non-peptide antagonists MK-329 and L-365,260. Brain Res. 1990 Sep 3;526(2):276–283. doi: 10.1016/0006-8993(90)91232-6. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Cortés R., Schalling M., Ceccatelli S., Pelto-Huikko M., Persson H., Villar M. J. Distribution patterns of CCK and CCK mRNA in some neuronal and non-neuronal tissues. Neuropeptides. 1991 Jul;19 (Suppl):31–43. doi: 10.1016/0143-4179(91)90081-s. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Herrera-Marschitz M., Seroogy K., Ju G., Staines W. A., Holets V., Schalling M., Ungerstedt U., Post C., Rehfeld J. F. Immunohistochemical studies on cholecystokinin (CCK)-immunoreactive neurons in the rat using sequence specific antisera and with special reference to the caudate nucleus and primary sensory neurons. J Chem Neuroanat. 1988 Jan-Feb;1(1):11–51. [PubMed] [Google Scholar]
- Ingram S. M., Krause R. G., 2nd, Baldino F., Jr, Skeen L. C., Lewis M. E. Neuronal localization of cholecystokinin mRNA in the rat brain by using in situ hybridization histochemistry. J Comp Neurol. 1989 Sep 8;287(2):260–272. doi: 10.1002/cne.902870209. [DOI] [PubMed] [Google Scholar]
- Lotti V. J., Chang R. S. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur J Pharmacol. 1989 Mar 21;162(2):273–280. doi: 10.1016/0014-2999(89)90290-2. [DOI] [PubMed] [Google Scholar]
- Marley P. D., Lightman S. L., Forsling M. L., Todd K., Goedert M., Rehfeld J. F., Emson P. C. Localization and actions of cholecystokinin in the rat pituitary neurointermediate lobe. Endocrinology. 1984 May;114(5):1902–1911. doi: 10.1210/endo-114-5-1902. [DOI] [PubMed] [Google Scholar]
- Meister B., Villar M. J., Ceccatelli S., Hökfelt T. Localization of chemical messengers in magnocellular neurons of the hypothalamic supraoptic and paraventricular nuclei: an immunohistochemical study using experimental manipulations. Neuroscience. 1990;37(3):603–633. doi: 10.1016/0306-4522(90)90094-k. [DOI] [PubMed] [Google Scholar]
- Meyer D. K., Krauss J. Dopamine modulates cholecystokinin release in neostriatum. Nature. 1983 Jan 27;301(5898):338–340. doi: 10.1038/301338a0. [DOI] [PubMed] [Google Scholar]
- Moran T. H., Robinson P. H., Goldrich M. S., McHugh P. R. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986 Jan 1;362(1):175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- Ravard S., Dourish C. T. Cholecystokinin and anxiety. Trends Pharmacol Sci. 1990 Jul;11(7):271–273. doi: 10.1016/0165-6147(90)90004-r. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Goltermann N., Larsson L. I., Emson P. M., Lee C. M. Gastrin and cholecystokinin in central and peripheral neurons. Fed Proc. 1979 Aug;38(9):2325–2329. [PubMed] [Google Scholar]
- Renaud L. P., Tang M., McCann M. J., Stricker E. M., Verbalis J. G. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987 Oct;253(4 Pt 2):R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- Richard P., Moos F., Freund-Mercier M. J. Central effects of oxytocin. Physiol Rev. 1991 Apr;71(2):331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- Saito A., Sankaran H., Goldfine I. D., Williams J. A. Cholecystokinin receptors in the brain: characterization and distribution. Science. 1980 Jun 6;208(4448):1155–1156. doi: 10.1126/science.6246582. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Montagnese C., Rodriguez F., Vincent J. D., Poulain D. A. Oxytocin induces morphological plasticity in the adult hypothalamo-neurohypophysial system. Nature. 1986 Aug 21;322(6081):738–740. doi: 10.1038/322738a0. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Lotstra F., Vandesande F., Dierickx K. Coexistence of cholecystokinin and oxytocin-neurophysin in some magnocellular hypothalamo-hypophyseal neurons. Cell Tissue Res. 1981;221(1):227–231. doi: 10.1007/BF00216585. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- Yang C. R., Bourque C. W., Renaud L. P. Dopamine D2 receptor activation depolarizes rat supraoptic neurones in hypothalamic explants. J Physiol. 1991 Nov;443:405–419. doi: 10.1113/jphysiol.1991.sp018840. [DOI] [PMC free article] [PubMed] [Google Scholar]


