Abstract
Histone methylation has emerged as an important mechanism for regulating the transcriptional accessibility of chromatin. Several methyltransferases have been shown to target histone amino-terminal tails and mark nucleosomes associated with either euchromatic or heterochromatic states. However, the biochemical machinery responsible for regulating histone methylation and integrating it with other cellular events has not been well characterized. We report here the purification, molecular identification, and genetic and biochemical characterization of the Set1 protein complex that is necessary for methylation of histone H3 at lysine residue 4 in Saccharomyces cerevisiae. The seven-member 363-kDa complex contains homologs of Drosophila melanogaster proteins Ash2 and Trithorax and Caenorhabditis elegans protein DPY-30, which are implicated in the maintenance of Hox gene expression and regulation of X chromosome dosage compensation, respectively. Mutations of Set1 protein comparable to those that disrupt developmental function of its Drosophila homolog Trithorax abrogate histone methylation in yeast. These studies suggest that epigenetic regulation of developmental and sex-specific gene expression are species-specific readouts for a common chromatin remodeling machinery associated mechanistically with histone methylation.
Covalent modification of histone amino-terminal tails regulates access to the underlying DNA and thus serves an important role in fine tuning gene expression (1). Histone modifications such as acetylation and phosphorylation facilitate transitions between chromatin states that are permissive or restrictive for transcription (2). More recently, histone methylation has emerged as an additional modification for recruitment of chromatin-associated proteins that determine transcriptional accessibility of genes. Several methyltransferases have been shown to target histone amino-terminal tails and mark nucleosomes associated with either euchromatic or heterochromatic states (3–5).
A subset of methyltransferases contains a highly conserved motif known as the SET domain. This motif was originally discovered in Drosophila proteins Su(var)3-9, Enhancer-of-zeste and Trithorax, which are involved in variegated or developmental gene expression (6). Although a functional role for the SET domain was not immediately evident, limited homology with plant methyltransferases prompted the discovery that Su(var)3-9 and its Schizosaccharomyces pombe homolog CLR4 are capable of transferring a methyl group onto Lys-9 of histone H3 (2). This methyl tag has subsequently been shown to mark nucleosomes that are associated with transcriptionally silenced genes in the Sch. pombe mating type locus (7), and the association of Su(var)3-9 homologs with the Rb corepressor complex suggests that Lys-9 methylation may also play a role in transcriptional repression of euchromatic genes (8).
Human SET domain-containing proteins such as MLL, a homolog of Drosophila Trithorax, NSD2, and NSD3 are essential for normal development and are also directly implicated in the pathogenesis of cancer (9–11). However, it remains to be determined what role these proteins serve in establishing and maintaining chromatin states and whether malfunction of their chromatin-modifying activity directly contributes to alterations in gene expression profiles leading to cancer (12). These efforts are likely to be compromised by the large number of SET domain proteins in mammalian cells (≈60), their possible functional redundancy, and their interactions with a variety of heterologous partners (13–18). Conversely, Saccharomyces cerevisiae contains only six SET-domain proteins. One of these is Set1p, which appears to play a role in silencing at telomeres and mating type loci (19) as well as in transcriptional activation of DNA repair genes (20). Its SET domain is 44% identical to that of Drosophila Trithorax, the founding member of the trxG transcriptional regulators required for the maintenance of homeotic gene expression in both mammals and Drosophila (21, 22). Studies have shown that some trxG proteins are components of large multiprotein complexes (23), but only members of the SWI/SNF family have been purified and biochemically characterized (24). Studies in yeast of this conserved chromatin-modifying machine, which mobilizes and reconfigures nucleosomes, have provided important insights into molecular mechanisms of developmental gene expression in higher eukaryotes.
We demonstrate in this report that Set1p is a component of a seven-member complex that contains homologs of Ash2, another trxG protein, as well as DPY-30, a protein involved in X-chromosome dosage compensation (25). The Set1p complex is required for histone H3 Lys-4 methylation, suggesting that there is a common mechanistic link between previously unrelated processes of chromatin regulation.
Methods
Protein Purification and Identification.
The Set1p complex was purified from the flow-through fraction of an RNA polymerase II affinity purification (26). Briefly, a protein extract was prepared from protease-deficient yeast by homogenization with glass beads and passed through a heparin-Sepharose column. Bound proteins were eluted with 0.6 M KCl, concentrated by ammonium sulfate precipitation, and taken up in a buffer containing 0.2 M ammonium sulfate. This fraction (50 ml) was gently mixed overnight at 4°C with Sepharose 4 Fast Flow (500 μl) conjugated with a polyol-sensitive (27) anti-Set1p monoclonal antibody. The Sepharose beads were collected by centrifugation (4,000 × g for 5 min) at 4°C then washed three times with 50 ml of wash buffer (150 mM ammonium sulfate/50 mM Tris, pH 7.4/1 mM EDTA/0.01% Nonidet P-40/10% glycerol/1 mM DTT) at 4°C and once with 1 ml of wash buffer at room temperature. Bound proteins were eluted in wash buffer (500 μl) containing 40% propylene glycol with gentle rotation for 15 min at 37°C. The affinity-purified complex was then subjected to gel filtration on a Superose 6 column (3.2/30) attached to a Smart System (Amersham Pharmacia) equilibrated with wash buffer. Approximately 60 μg of more than 95% pure Set1p complex were obtained from 50 liters of starting yeast culture, constituting an approximate yield of 30%. The purified and trichloroacetic acid-precipitated Set1p complex was fractionated on an SDS 8–16% polyacrylamide gradient or 10% polyacrylamide gel. After staining with Coomassie blue, individual protein bands were cut out and dried, and their identities were determined based on the masses of constituent peptides as measured by matrix-assisted laser desorption time-of-flight mass spectrometry (28). Sizing of the unpurified complex was performed by gel filtration on a Superose 6 column under conditions described above. Mouse antisera specific for Set1p complex components were raised against purified maltose binding protein fusion proteins and used for Western blots at 1:500 dilution. Secondary antibodies consisted of goat anti-mouse IgG (Accurate Chemical and Scientific, Westbury, NY) used at 1:5,000 dilution.
Yeast Strains and Procedures.
Telomere silencing was evaluated after disruption of individual ORFs in the strain UCC3537 [MATa ura3-52 lys2-801 ade2-101 leu2-Δ1 trp1-Δ63 his3-Δ200 adh4∷URA3 (URA3 at VIIL) DIA5-1 (ADE2 at VR)] (a gift of Dan Gottschling, Fred Hutchinson Cancer Research Center, Seattle) carrying URA3 and ADE2 reporters at the telomeres of the left arm of chromosome VII and the right arm of chromosome V, respectively. The entire ORF for each gene encoding Set1p complex components (and SIR2 as a control) was replaced by a G418-resistance cassette by using standard procedures. Genomic DNA fragments extending 200 bp upstream from the start codon and 200 bp downstream from the stop codon of each ORF were cloned into pRS315 and used for complementation.
Yeast strains deficient for Set1p complex components on the UCC3537 background were tested for sensitivity to formamide (2.5%), rapamycin (0.1 ng/ml), and chlorpromazine (30 μM) [on Hartwell Complete (HC) Leu− plates] and for flocculation studies (HC complete Leu− liquid medium). Homozygous diploid mutants on the S288C background (YM4174 MATa ura3-52 lys2-801 ade2-101 leu2-Δ1 trp1 his3-Δ200 TYR MET can1, MATα ura3-52 lys2-801 ade2-101 leu2-Δ1 TRP his3-Δ200 tyr MET can1) were tested for growth on yeast extract/peptone (YP) glucose (2%), YP sucrose (2%), YP raffinose (2%), and YP galactose (2%) plates containing 20 μg/ml ethidium bromide. The same strains were tested for sporulation efficiency by using standard protocols. Homothallic switching endonuclease (HO) promoter activity was tested by measuring β-galactosidase activity in haploid S288C strains after crossing of the relevant mutant strains with CY114 (MATa HO–lacZ ura3-52 lys2-801 ade2-101 leu2-Δ1 his3-Δ200) (gift of Craig Peterson, University of Massachusetts Medical School, Worcester, MA) and tetrad dissection.
Histone Methylation Studies.
Core histone octamers were purified as described (29) from wild-type or mutant yeast strains (UCC3537 derivatives) grown in YP dextrose to OD 1.0 at 600 nm. Western blots were performed by using an anti-methyllysine-4 H3 antiserum (United States Biochemical) at 1:2000 dilution. Horseradish peroxidase-conjugated anti-rabbit IgG secondary antibodies were used at 1:5000 dilution. The SET domain requirement for histone H3 Lys-4 methylation was analyzed in a UCC3537 derivative strain (set1:HIS3) complemented with full-length or mutated versions of SET1 fused to the carboxyl terminus of green fluorescent protein (GFP) expressed from the ACT1 promoter derived from pRB2138 (30). Constructs were sequenced to confirm the presence of engineered mutations and GFP fusion protein expression was assessed by Western blotting and fluorescence microscopy. Cells were grown in HC leu− medium, and histone methylation was assessed as described above.
Results and Discussion
Purification of a Set1p Complex from S. cerevisiae.
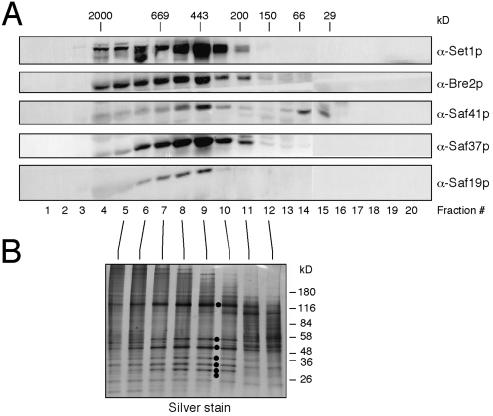
To characterize the role of Set1p as potentially part of a multiprotein complex, a clarified crude extract of yeast was passed through a Superose 6 sizing column. Western blot analysis revealed a Set1p peak at 440 kDa (Fig. 1A), which is considerably greater than the predicted 124-kDa molecular mass of monomeric Set1p, suggesting that it was associated with additional proteins in vivo. Set1p and associated factors were purified by affinity chromatography using a polyol-sensitive, anti-Set1p monoclonal antibody generated following the protocol of Thompson and Burgess (27). Elution of Set1p from the affinity column with propylene glycol instead of denaturants allowed further purification by gel filtration. Sizing analysis of affinity-purified Set1p revealed a molecular mass identical to that observed in the crude extract (Fig. 1), suggesting that the major components of the complex remain associated during purification.
Figure 1.
Purification of a Set1p-containing protein complex from S. cerevisiae. (A) A crude extract of S. cerevisiae proteins was passed through a gel filtration column, and the resulting fractions were analyzed by Western blot analysis using an anti-Set1p monoclonal antibody or mouse antisera specific for Set1p complex components Bre2p, Saf41p, Saf37p, and Saf19p (indicated to the right of respective panels). (B) Silver-stained SDS 4–16% gradient PAGE of gel-filtration fractions of immunopurified Set1p complex after propylene glycol-assisted release from anti-Set1p affinity column. Dots indicate complex components from top to bottom: Set1p, Bre2p, Saf49p, Saf41p, Saf37p, Saf35p, and Saf19p, the products of the SET1 gene and ORFs YLR015W, YAR003W, YPL138C, YKL018W, YBR175W, and YDR469W, respectively.
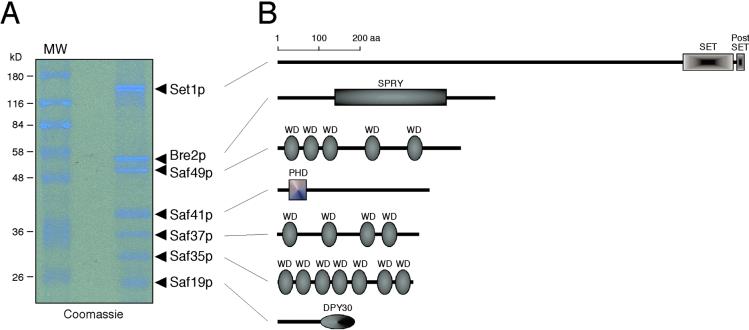
Denaturing gel electrophoretic analysis of the purified Set1p complex revealed the presence of seven polypeptides. These were identified by mass spectrometry as Set1p and six previously uncharacterized proteins encoded by YLR015W(BRE2), YAR003W, YPL138C, YKL018W, YBR175W, and YDR469W (Fig. 1B and Fig. 2). These Set1p-associated factors are hereafter referred to as Bre2p, Saf49p, Saf41p, Saf37p, Saf35p, and Saf19p, respectively. Their predicted molecular masses totaled 363 kDa, which is compatible with the apparent mass of the Set1p complex determined by gel-filtration chromatography. Coomassie blue-stained gels of the purified complex suggested that each component was present in stoichiometric amounts, although we cannot exclude that one or more of the components may be present in nonequimolar ratios, which would further increase the mass of the complex. Antisera generated against four of the six components showed that they cofractionated with Set1p in crude extract (Fig. 1A). In accordance with our observations, genomewide two-hybrid studies (31) identified interactions between Saf49p and Saf35p as well as between Bre2p and Saf19p. Taken together, these observations establish that Set1p is a component of a previously undescribed multiprotein complex.
Figure 2.
Subunit composition of the Set1p complex. (A) Coomassie blue-stained SDS/10% PAGE analysis of gel-filtration fraction 9 (see Fig. 1) used for recovery of complex components for mass spectrometric analysis. (B) Schematic representation of the domain structure of the Set1p complex components. Domain assignments were made by using the smart tool (32), except for the DPY-30 homology domain, which was assigned based on similarities between S. cerevisiae, Caenorhabditis elegans, D. melanogaster, and Homo sapiens protein sequences.
The Set1p Complex Contains Homologs of D. melanogaster Ash2 and C. elegans DPY-30.
Sequence alignment and motif analysis (32) showed that Bre2p contains an SPRY domain (Fig. 2A), a motif of unknown function that is present in a wide variety of proteins (33). Bre2p is most closely related to Drosophila Ash2, a trxG gene product required for imaginal disk pattern formation (34). However, Bre2p lacks a highly conserved PHD finger, which is found in Ash2 orthologs of D. melanogaster, C. elegans, and Sch. pombe (35). Interestingly, another component of the Set1p complex, Saf41p, contains a PHD finger similar to that of Yng2p, a component of the NuA4 histone acetyltransferase complex (36) and its human homolog, the Ing1 tumor suppressor protein (37). Thus, it is possible that Bre2p and Saf41p together constitute a bipartite functional homolog of Ash2. The smallest member of the complex, Saf19p, is an apparent ortholog of C. elegans DPY-30, which is required for X-chromosome dosage compensation in hermaphrodite worms (38). Although DPY-30 itself has a diffuse nuclear localization, it has been shown to be essential for the sex-specific association of other dosage compensation factors with the X chromosome. The mechanism of DPY-30 activity is unknown (39). Finally, the Set1p complex also contains three WD-repeat-containing proteins (Saf49p, Saf37p, and Saf35p; ref. 40) that contain no other recognizable motifs (Fig. 2A).
Yeast Deficient for Set1p Complex Components Display Overlapping Phenotypes.
With the exception of the WD-repeat-containing Saf37p, all complex components were nonessential for vegetative growth in either the haploid or diploid state. Homozygous diploid mutants sporulated with the exception of the set1 strain. Yeast strains singly deficient for the nonessential components displayed similar flocculation phenotypes and comparable sensitivity to formamide, chlorpromazine, and rapamycin (Table 1). Cells lacking Saf41p, the PHD finger protein, consistently displayed a less severe phenotype under these conditions. Strains deficient for Set1p complex components grew on nonglucose carbon sources and were able to activate the HO promoter, in contrast to yeast mutant for Swi2, another trxG-related protein. Synthetic effects on growth were not observed for compound mutants deficient for two or more nonessential components (data not shown). The observed epistasis suggests that the components function in a common genetic pathway and is consistent with their participation in a biochemical complex.
Table 1.
Summary of phenotypes observed in yeast strains mutant for Set1p complex components
| wt | set1 | bre2 | saf49 | saf41 | saf35 | saf19 | |
|---|---|---|---|---|---|---|---|
| Flocculation | − | + | + | + | +/− | + | + |
| Formamide sensitive | − | + | + | + | +/− | + | + |
| Chlorpromazine sensitive | − | + | + | + | +/− | + | + |
| Rapamycin sensitive | − | + | + | + | +/− | + | + |
| Histone H3 lys-4 methylation | 100% | 0 | 10% | 0 | 50% | 0 | 10% |
wt, wild type.
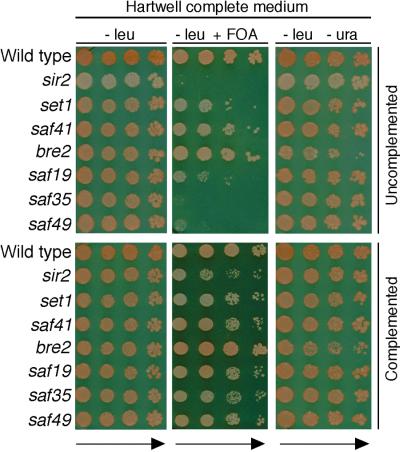
In addition to a potential role in activating transcription, Set1p has also been implicated in silencing at telomeres and mating-type loci (19). Consistent with previous studies of set1 strains, yeast deficient for other complex components, with the exception of bre2, showed various levels of derepression of a telomeric URA3 marker as evidenced by 5-fluoroorotic acid (FOA) sensitivity and normal growth on Ura− plates (Fig. 3). In contrast, the bre2 strain displayed enhanced FOA resistance and partial uracil auxotrophy (Fig. 3). This phenotype likely reflects that BRE2 shares its promoter with PPR1, which codes for the activator of URA3. Thus, full-length disruption of BRE2 may lead to alterations of the PPR1 promoter and reduced levels of Ppr1p. Based on these observations, we suggest that the observed telomeric silencing deficiencies in strains lacking Set1p complex components might be URA3-specific events reflecting compensatory changes at the PPR1-BRE2 promoter. This theory is supported by our inability to detect silencing defects by using an ADE2 telomeric marker (Fig. 3). Although other yeast orthologs of trxG proteins, such as Swi/Snf, have been implicated in both activating and repressive transcriptional processes, it remains to be determined whether a role for the Set1p complex in silencing is direct or indirect.
Figure 3.
Telomere silencing defects in yeast strains deficient for Set1p complex components. The plating efficiencies and sectoring of strains with URA3 and ADE2 present at telomeric sites (UCC3537) were assessed in yeast strains deficient for Set1p complex components or SIR2 (Upper) transformed with pRS315 vector and after complementation with pRS315 carrying the respective exogenous genes (Lower). Equivalent aliquots of 10-fold serial dilutions were plated on synthetic medium lacking leucine in the presence or absence of FOA or uracil. Note that the saf37 mutant could not be studied because it is nonviable.
The Set1p Complex Is Required for Methylation of Histone H3 on Lys-4.
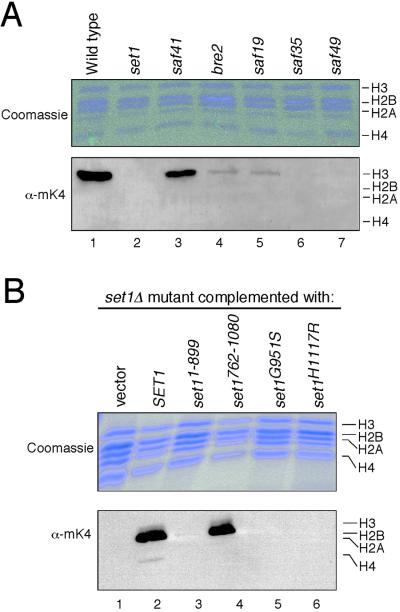
Despite extensive genetic studies, the biochemical functions of most SET domain proteins are unknown. Recently, the SET domain protein SUV39H1 was shown to be a histone H3-specific methyltransferase (3) whose methylation of Lys-9 recruits heterochromatin protein HP1 in heterochromatic silencing (41, 42) and euchromatic gene repression (8). Unlike higher eukaryotes and Sch. pombe, S. cerevisiae has no ortholog of SUV39H1 and lacks methylation of histone H3 Lys-9. However, lysine methylation is observed at residue 4 within the amino-terminal tail of histone H3 in S. cerevisiae (43). We therefore evaluated whether yeast deficient for members of the Set1p complex displayed alterations in histone H3 Lys-4 methylation. Histones were purified from various mutant strains and examined by Western blot analysis using an antiserum specific for methyllysine-4 of histone H3. Complete loss of H3 Lys-4 methylation was observed in the set1 strain as well as strains deficient for WD-repeat proteins Saf49p and Saf35p. Greater than 90% decrease in Lys-4-specific methylation was observed in the bre2 and saf19 strains, versus an approximate 50% decrease in the strain deficient for PHD protein Saf41p (Fig. 4A). Relative preservation of histone methylation in the latter correlates with its milder phenotypic readout in genetic assays (Table 1). Loss of histone H3 lys4 methylation in the set1 strain was rescued by Set1p, but not by mutant Set1p lacking the carboxyl-terminal SET domain (set11–899). H3 lys4 methylation was restored by a C-terminal portion of Set1p containing the SET domain and flanking sequences (set1762–1080). However, methylation was not restored by comparable constructs harboring mutations in SET domain residues that are conserved in plant methyltransferases and essential for methyltransferase activity of SUV39H1 (set1H1117R) (3) or the embryonic function of Drosophila Trithorax (set1G951S) (Fig. 4B; refs. 6 and 44). These results are consistent with a role for Set1p as a histone methyltransferase; however, we have not observed methylation of nucleosomes or histone tails by the purified Set1p complex or recombinant Set1p in vitro. This observation suggests that its activity is constrained by undefined enzymatic requirements or, less likely, that it plays an indirect role in histone methylation. Nevertheless, our data indicate that the Set1p complex is essential for Lys-4 methylation of histone H3 in S. cerevisiae and implicate the SET domain of Set1p (and its MLL homolog) as likely histone methyltransferases.
Figure 4.
The Set1p complex is required for Lys-4 methylation of histone H3 in vivo. (A) Core histones purified from either wild-type (lane 1) or mutant yeast strains (lanes 2–7) deficient for Set1p complex components (indicated above gel lanes) were analyzed by Coomassie blue-stained SDS/PAGE (Upper) and Western blotting using an anti-methyllysine-4 antiserum (Lower). (B) Core histones were purified from set1 strains expressing the constructs indicated above the gel lanes and analyzed for histone H3 Lys-4 methylation by SDS/PAGE and Western blot analysis.
The Set1p Complex Suggests a Mechanistic Model for Developmental and Sex-Specific Epigenetic Regulation.
These observations suggest a model in which Trithorax-like SET domain proteins exert their influence on chromatin through histone methylation, whereas Ash2-like trxG proteins apparently modulate this activity by working in conjunction with DPY-30-like molecules. Based on the physical association of Bre2p (Ash2-related) and Saf19p (DPY-30-related), and the genetic similarities of their mutant phenotypes, we propose that maintenance of Hox gene expression in D. melanogaster (Ash2-dependent) and regulation of X chromosome dosage compensation in C. elegans (DPY-30-dependent) are species-specific readouts for a common chromatin remodeling machinery. Besides causing XX-specific lethality, dpy-30 mutations in XO animals cause developmental delay, small body size, an inability to mate, and abnormal tail morphology (45). These phenotypes suggest a broader role for DYP-30, and more detailed study of Hox gene expression patterns is warranted in dpy-30 mutants.
WD-repeat-containing proteins of the Set1p complex are likely to target its methyltransferase activity to substrate histone H3. This suggestion is based on (i) the complete loss of histone H3 Lys-4 methylation in strains deficient for WD-repeat proteins Saf49p and Saf35p; (ii) the fact that these proteins physically interact in a two-hybrid screen (31); and (iii) the structural similarity of Saf35p to Cac3p and Hat2p, two other seven-WD-repeat-containing proteins that bind histones and are components of other chromatin-modifying complexes (46). The role of Saf37p, a WD-repeat protein that is the only essential member of the complex, is uncertain, but our data show it to be present exclusively in elution fractions from the sizing column that contain Set1p. This finding argues against its participation in other complexes, or in functions unrelated to Set1p.
A potential role for histone H3 Lys-4 methylation effected by the Set1p machinery is suggested by recent studies in Sch. pombe that associate this modification with transcriptionally competent euchromatic regions (7). It has also been shown that histone H3 Lys-4 methylation is conserved from yeast to humans (43). In this context, the presence of Ash2 and Trithorax homologs in a single complex and their requirement for histone methylation in vivo provide a molecular basis for their genetic interaction in Drosophila and suggest a mechanism for maintenance of developmental gene expression.
Acknowledgments
We extend special thanks to Nancy Thompson and Richard Burgess for advice concerning the preparation and testing of polyol-sensitive antibodies, Bich-Tien Rouse for hybridoma generation and culture, and Barbara Davis for large-scale preparation of yeast extracts. We thank Dan Gottschling, Tom Petes, Craig Peterson, Lorraine Pillus, Katja Schwartz, and David Botstein for yeast strains and plasmids, and Yi Zhang for valuable advice and reagents for histone methylase assays. These studies were supported by National Institutes of Health Grant CA55029.
Abbreviations
- HO
homothallic switching endonuclease
- FOA
5-fluoroorotic acid
References
- 1.Jenuwein T, Allis C D. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 2.Kingston R E, Narlikar G J. Genes Dev. 1999;13:2239–2252. doi: 10.1101/gad.13.18.2339. [DOI] [PubMed] [Google Scholar]
- 3.Rea S, Eisenhaber F, O'Carroll D, Strahl B D, Sun Z W, Schmid M, Opravil S, Mechtler K, Ponting C P, Allis C D, et al. Nature (London) 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Ma H, Hong H, Koh S S, Huang S M, Schurter B T, Aswad D W, Stallcup M R. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Huang Z Q, Xia L, Feng Q, Erdjument-Bromage H, Strahl B D, Briggs S D, Allis C D, Wong J, Tempst P, et al. Science. 2001;293:853–857. doi: 10.1126/science.1060781. [DOI] [PubMed] [Google Scholar]
- 6.Stassen M J, Bailey D, Nelson S, Chinwalla V, Harte P J. Mech Dev. 1995;52:209–223. doi: 10.1016/0925-4773(95)00402-m. [DOI] [PubMed] [Google Scholar]
- 7.Noma K, Allis C D, Grewal S I S. Science. 2001;293:1150–1155. doi: 10.1126/science.1064150. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen S J, Schneider R, Bauer U M, Bannister A J, Morrison A, O'Carroll D, Firestein R, Cleary M, Jenuwein T, Herrera R E, et al. Nature (London) 2001;412:561–565. doi: 10.1038/35087620. [DOI] [PubMed] [Google Scholar]
- 9.Tkachuk D C, Kohler S, Cleary M L. Cell. 1992;71:691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 10.Stec I, Wright T J, van Ommen G J, de Boer P A, van Haeringen A, Moorman A F, Altherr M R, den Dunnen J T. Hum Mol Genet. 1998;7:1071–1082. doi: 10.1093/hmg/7.7.1071. [DOI] [PubMed] [Google Scholar]
- 11.Angrand P O, Apiou F, Stewart A F, Dutrillaux B, Losson R, Chambon P. Genomics. 2001;74:79–88. doi: 10.1006/geno.2001.6524. [DOI] [PubMed] [Google Scholar]
- 12.Johnson S, Pillus L. Curr Opin Gen Dev. 1999;9:175–184. doi: 10.1016/S0959-437X(99)80027-6. [DOI] [PubMed] [Google Scholar]
- 13.Jenuwein T, Laible G, Dorn R, Reuter G. Cell Mol Life Sci. 1998;54:80–93. doi: 10.1007/s000180050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corda Y, Schramke V, Longhese M P, Smokvina T, Paciotti V, Brevet V, Gilson E, Geli V. Nat Genet. 1999;21:204–208. doi: 10.1038/5991. [DOI] [PubMed] [Google Scholar]
- 15.Rozovskaia T, Tillib S, Smith S, Sedkov Y, Rozenblatt-Rosen O, Petruk S, Yano T, Nakamura T, Ben-Simchon L, Gildea J, et al. Mol Cell Biol. 1999;19:6441–6447. doi: 10.1128/mcb.19.9.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozenblatt-Rosen O, Rozovskaia T, Burakov D, Sedkov Y, Tillib S, Blechman J, Nakamura T, Croce C M, Mazo A, Canaani E. Proc Natl Acad Sci USA. 1998;95:4152–4157. doi: 10.1073/pnas.95.8.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso C, Timsit S, Villard L, Khrestchatisky M, Fontes M, Colleaux L. Hum Mol Genet. 1998;7:679–684. doi: 10.1093/hmg/7.4.679. [DOI] [PubMed] [Google Scholar]
- 18.Cui X, De Vivo I, Slany R, Miyamoto A, Firestein R, Cleary M L. Nat Genet. 1998;18:331–337. doi: 10.1038/ng0498-331. [DOI] [PubMed] [Google Scholar]
- 19.Nislow C, Ray E, Pillus L. Mol Biol Cell. 1997;8:2421–2436. doi: 10.1091/mbc.8.12.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schramke V, Neecke H, Brevet V, Corda Y, Lucchini G, Longhese M P, Gilson E, Geli V. Genes Dev. 2001;15:1845–1858. doi: 10.1101/gad.193901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennison J A. Annu Rev Genet. 1995;29:289–303. doi: 10.1146/annurev.ge.29.120195.001445. [DOI] [PubMed] [Google Scholar]
- 22.Francis N J, Kingston R E. Nat Rev. 2001;2:409–421. doi: 10.1038/35073039. [DOI] [PubMed] [Google Scholar]
- 23.Papoulas O, Beek S J, Moseley S L, McCallum C M, Sarte M, Shearn A, Tamkun J W. Development (Cambridge, UK) 1998;125:3955–3966. doi: 10.1242/dev.125.20.3955. [DOI] [PubMed] [Google Scholar]
- 24.Vignali M, Hassan A H, Neely K E, Workman J L. Mol Cell Biol. 2000;20:1899–1910. doi: 10.1128/mcb.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer B J. Trends Genet. 2000;16:247–253. doi: 10.1016/s0168-9525(00)02004-7. [DOI] [PubMed] [Google Scholar]
- 26.Edwards A M, Darst S A, Feaver W J, Thompson N E, Burgess R R, Kornberg R D. Proc Natl Acad Sci USA. 1990;87:2122–2126. doi: 10.1073/pnas.87.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson N E, Burgess R R. Methods Enzymol. 1996;274:513–526. doi: 10.1016/s0076-6879(96)74041-7. [DOI] [PubMed] [Google Scholar]
- 28.Roepstorff P. EXS. 2000;88:81–97. doi: 10.1007/978-3-0348-8458-7_6. [DOI] [PubMed] [Google Scholar]
- 29.Edmondson D G, Smith M M, Roth S Y. Genes Dev. 1996;10:1247–1259. doi: 10.1101/gad.10.10.1247. [DOI] [PubMed] [Google Scholar]
- 30.Doyle T, Botstein D. Proc Natl Acad Sci USA. 1996;93:3886–3891. doi: 10.1073/pnas.93.9.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uetz P, Giot L, Cagney G, Mansfield T A, Judson R S, Knight J R, Lockshon D, Narayan V, Srinivasan M, Pochart P, et al. Nature (London) 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 32.Schultz J, Copley R R, Doerks T, Ponting C P, Bork P. Nucleic Acids Res. 2000;28:231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponting C, Schultz J, Bork P. Trends Biochem Sci. 1997;22:193–194. doi: 10.1016/s0968-0004(97)01049-9. [DOI] [PubMed] [Google Scholar]
- 34.Adamson A L, Shearn A. Genetics. 1996;144:621–633. doi: 10.1093/genetics/144.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aashland R, Gibson T J, Stewart A F. Trends Biochem Sci. 1995;20:56–59. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- 36.Loewith R, Meijer M, Lees-Miller S P, Riabowol K, Young D. Mol Cell Biol. 2000;20:3807–3816. doi: 10.1128/mcb.20.11.3807-3816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garkavtsev I, Kazarov A, Gudkov A, Riabowol K. Nat Genet. 1996;14:415–420. doi: 10.1038/ng1296-415. [DOI] [PubMed] [Google Scholar]
- 38.Hsu D R, Chuang P, Meyer B J. Development (Cambridge, UK) 1995;121:3323–3334. doi: 10.1242/dev.121.10.3323. [DOI] [PubMed] [Google Scholar]
- 39.Davis T L, Meyer B J. Development (Cambridge, UK) 1997;124:1019–1031. doi: 10.1242/dev.124.5.1019. [DOI] [PubMed] [Google Scholar]
- 40.Smith TF, Gaitatzes C, Saxena K, Neer E J. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 41.Bannister A J, Zegerman P, Partridge J F, Miska E A, Thomas J O, Allshire R C, Kouzarides T. Nature (London) 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 42.Lachner M, O'Carroll D, Rea S S, Mechtler K, Jenuwein T. Nature (London) 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 43.Strahl B D, Ohba R, Cook R G, Allis C D. Proc Natl Acad Sci USA. 1999;96:14967–14972. doi: 10.1073/pnas.96.26.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katsani K R, Arredondo J J, Kal A J, Verrijzer C P. Genes Dev. 2001;15:2197–2202. doi: 10.1101/gad.201901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu D R, Meyer B J. Genetics. 1994;137:999–1018. doi: 10.1093/genetics/137.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman P D, Cohen J L, Osley M A. Mol Cell Biol. 1998;18:4793–4806. doi: 10.1128/mcb.18.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]