Abstract
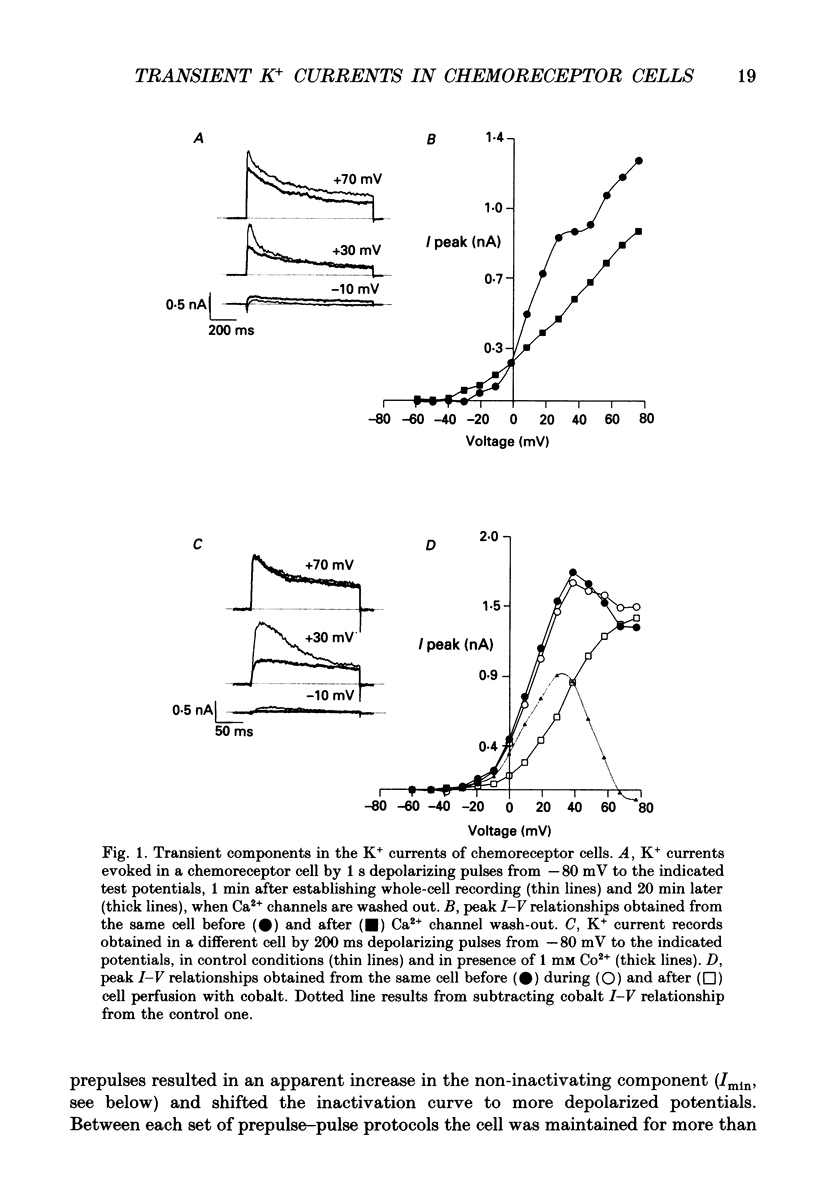
1. Adult rabbit carotid body chemoreceptor cells, enzymatically dispersed and short-term cultured, exhibit an inactivating outward K+ current that is reversibly inhibited by low PO2. In the present work we have characterized the biophysical and pharmacological properties of this current using the whole-cell voltage clamp recording technique. 2. Inactivating current was recorded after blockage of Ca2+ currents with extracellular Co2+, Cd2+, or after complete washing out of Ca2+ channels. 3. The threshold of activation of this inactivating current was about -40 mV. Current activated very quickly (mean rise time 4.8 +/- 0.42 ms at +60 mV) but inactivated more slowly. Inactivation was well fitted by two exponentials with time constants of 79.7 +/- 6.6 and 824 +/- 42.8 ms (at +40 mV). The inactivation process showed a little voltage dependence. 4. The steady-state inactivation was well fitted by a Boltzman function. Inactivation was fully removed at potentials negative to -80 mV and was complete at voltages near -10 mV; 50% inactivation occurred at -41 mV. 5. Recovery from inactivation had several components and was voltage dependent. Initial recovery was fast, but full recovery, even at -100 mV, required more than 30 s. 6. Inactivating current was selectively blocked by 4-aminopyridine (4-AP), in a dose-dependent manner (IC50, 0.2 mM). The duration of chemoreceptor cells action potentials was augmented by 1 mM 4-AP from 2.3 +/- 0.36 to 7.0 +/- 0.25 ms at 0 mV. Tetraethylamonium (TEA), at concentrations above 5 mM, blocked inactivating and non-inactivating components of the whole K+ current. 7. Inactivating current was modulated by cyclic AMP (cAMP). Bath application of 2 mM dibutyryl cAMP reduced peak amplitude by 18.7 +/- 2.9% (at +30 mV) and slowed down the rise time of the current. The effect was not voltage dependent. Forskolin (10-20 microM) also affected inactivating current, by accelerating the inactivation process. In the same preparations neither dibutyryl cAMP nor forskolin affected Ca2+ currents. 8. It is concluded that modulation of K+ channels by cAMP might play a physiological role potentiating the low PO2 inhibition of K+ channels.
Full text
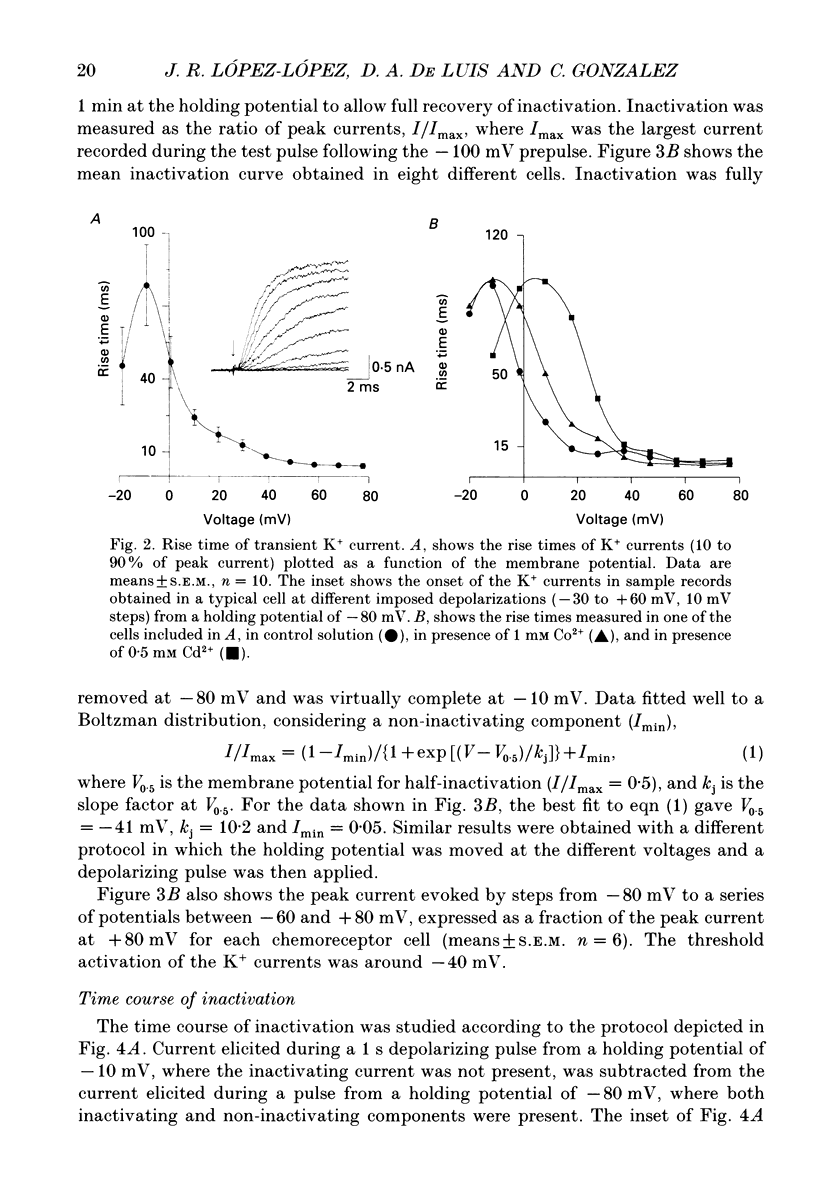
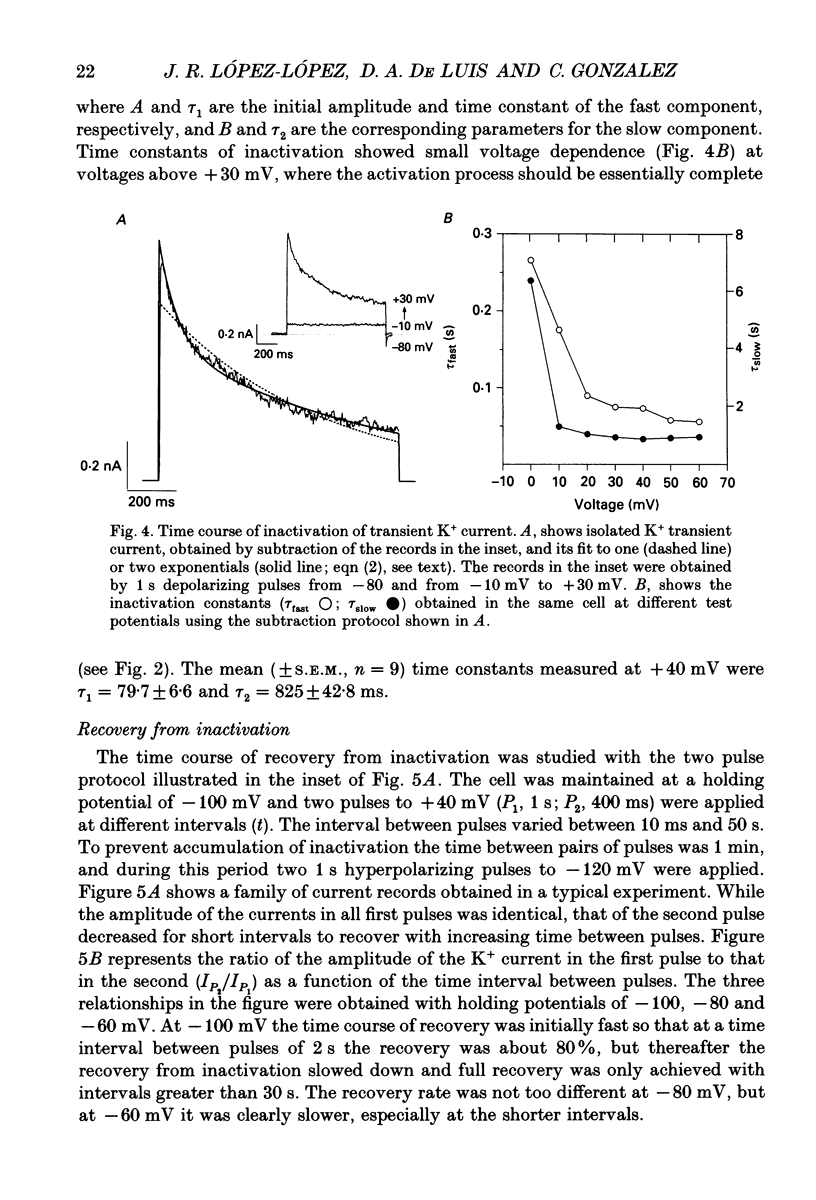
PDF



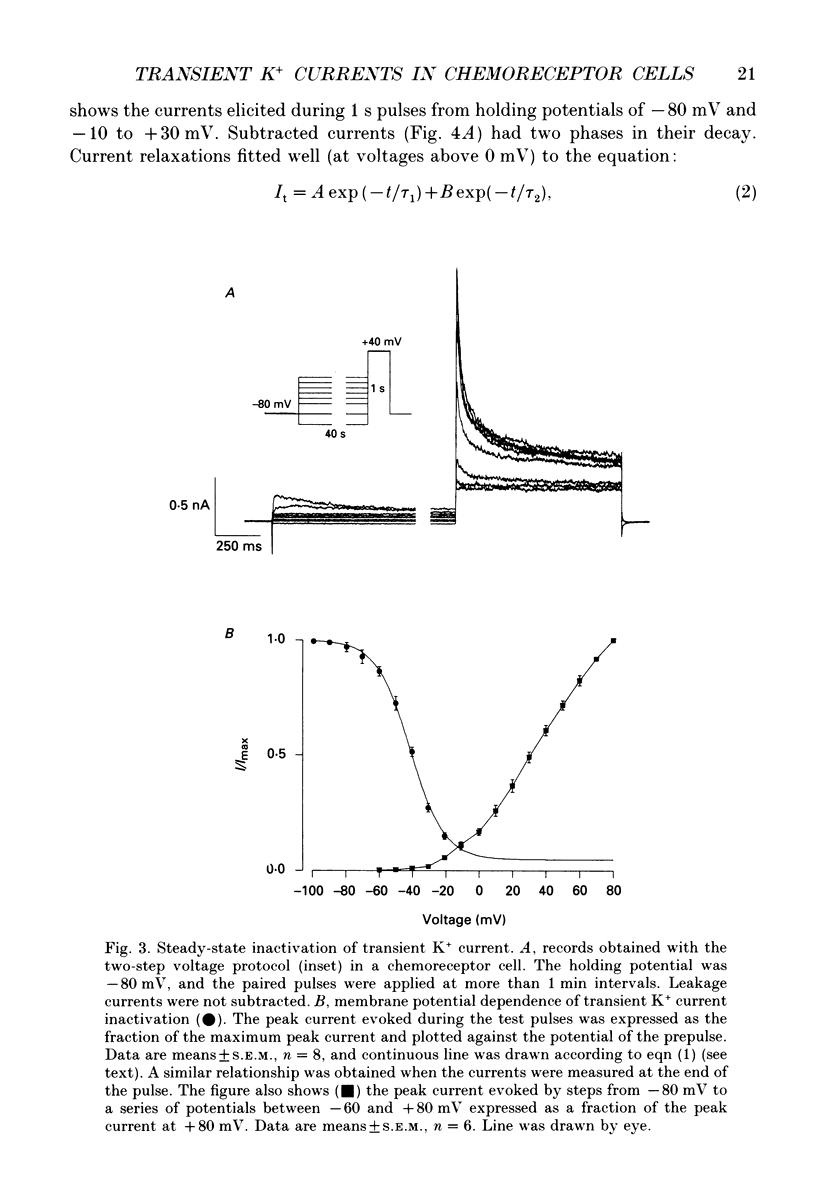





Selected References
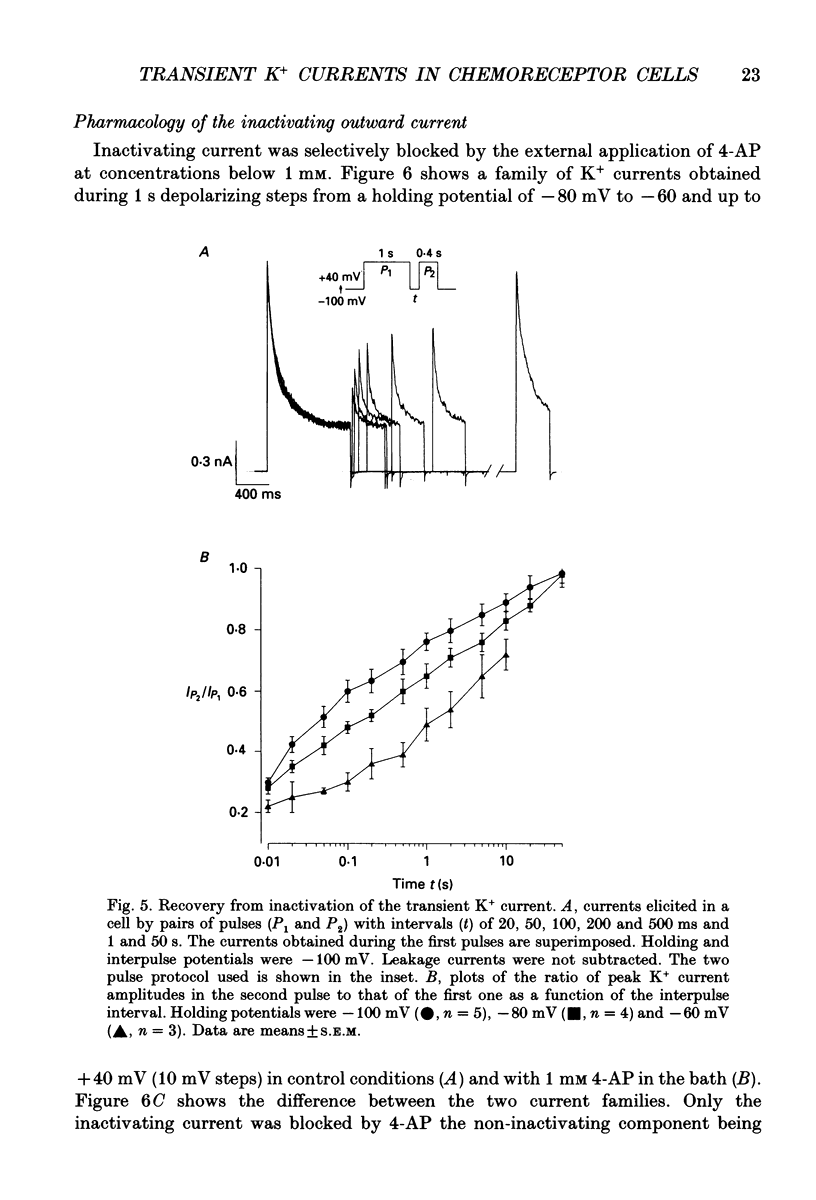
These references are in PubMed. This may not be the complete list of references from this article.
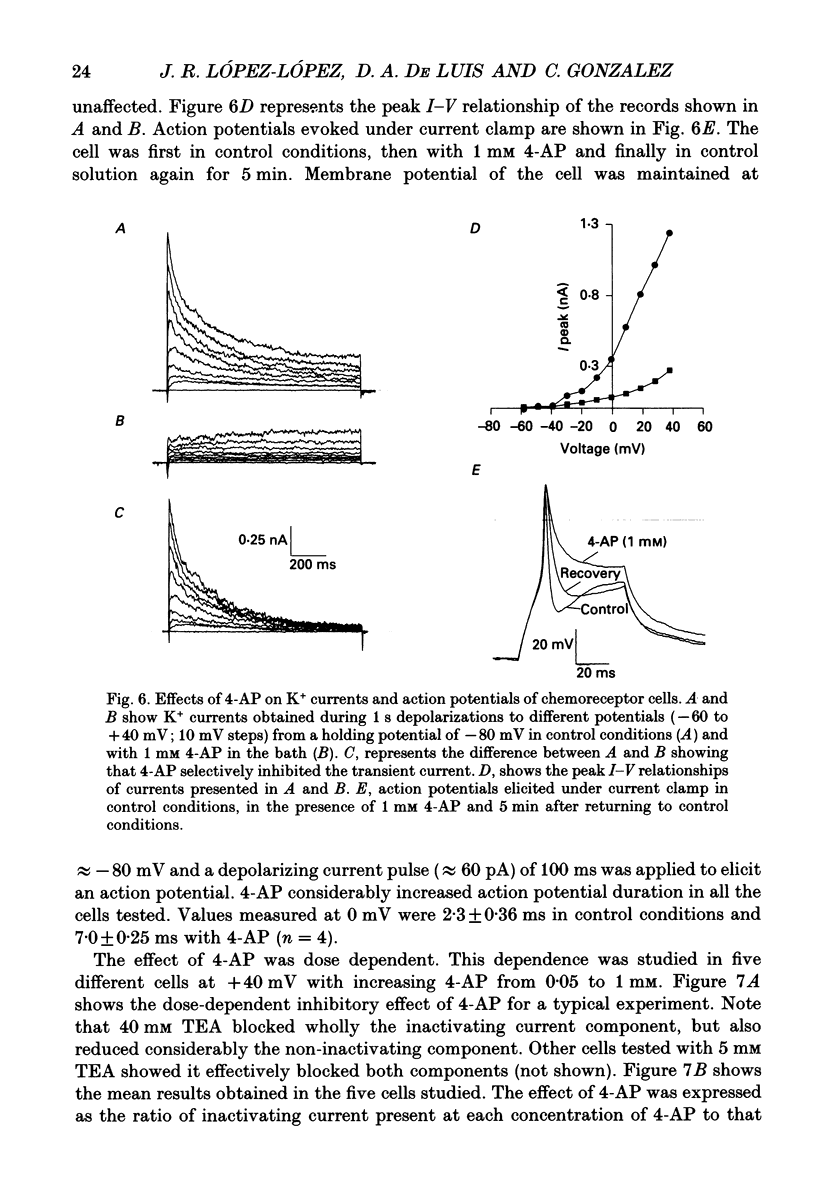
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
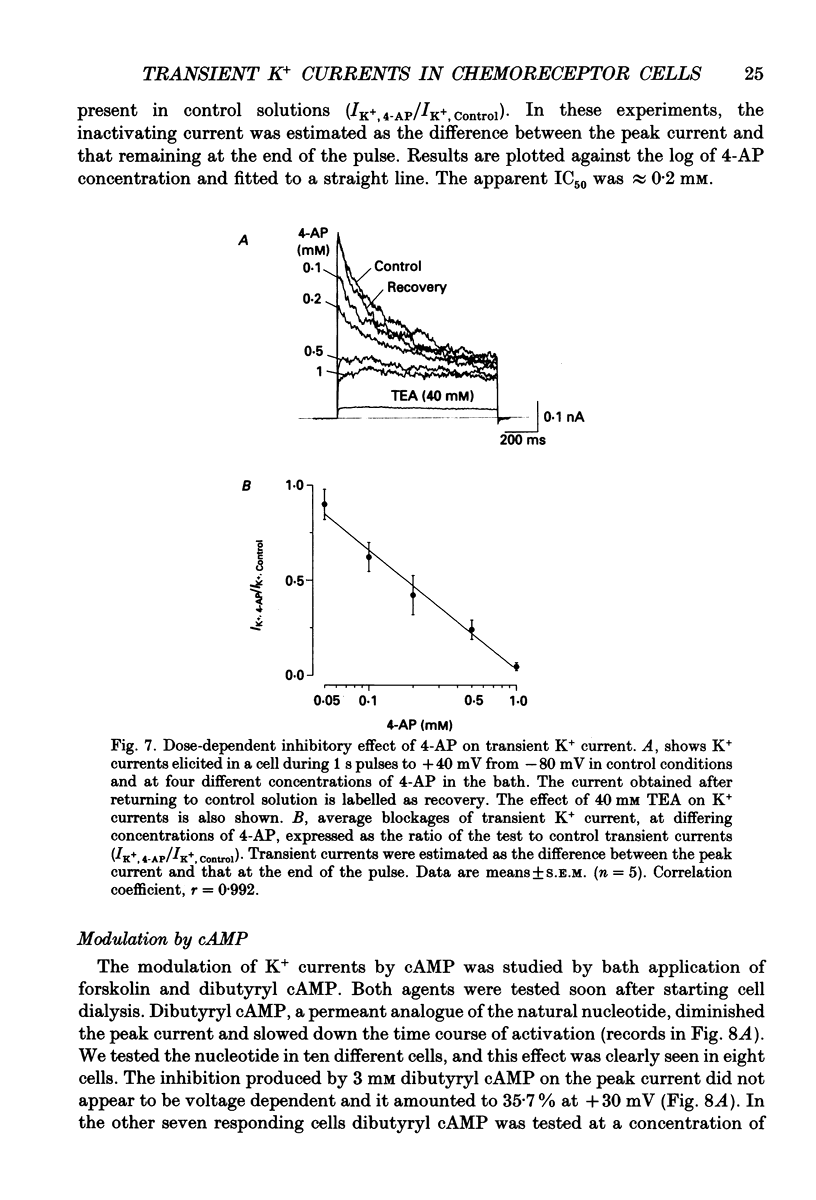
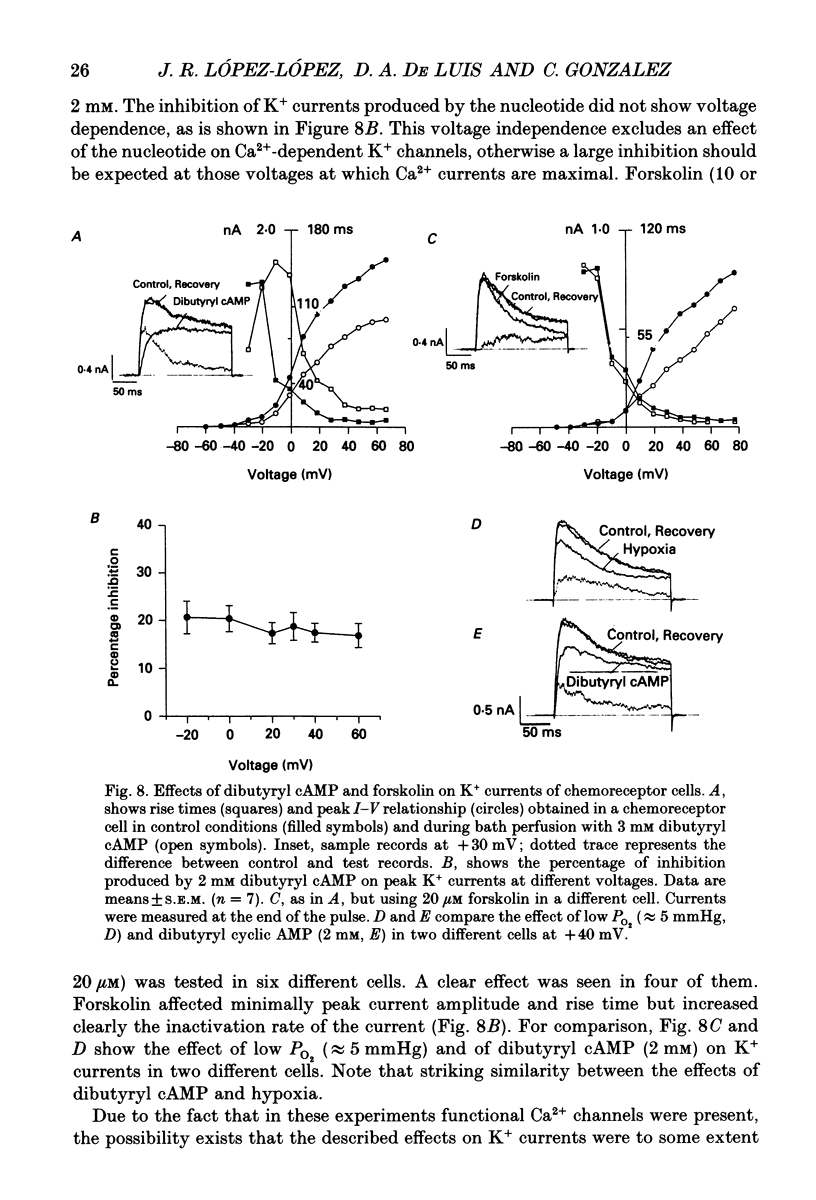
- Belluzzi O., Sacchi O., Wanke E. Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clamp. J Physiol. 1985 Jan;358:109–129. doi: 10.1113/jphysiol.1985.sp015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M., Nunoki K. Molecular properties of dihydropyridine-sensitive calcium channels. Ann N Y Acad Sci. 1989;560:1–14. doi: 10.1111/j.1749-6632.1989.tb24074.x. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Giles W. R., Imaizumi Y. Properties of the transient outward current in rabbit atrial cells. J Physiol. 1988 Nov;405:147–168. doi: 10.1113/jphysiol.1988.sp017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Shrier A. Inactivation of A currents and A channels on rat nodose neurons in culture. J Gen Physiol. 1989 Nov;94(5):881–910. doi: 10.1085/jgp.94.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T. E. State-dependent inactivation of K+ currents in rat type II alveolar epithelial cells. J Gen Physiol. 1990 Apr;95(4):617–646. doi: 10.1085/jgp.95.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., Caddy K. W., Kirby G. C., Patterson D. L., Ponte J., Biscoe T. J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988 Jul;26(1):291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina M. D., López-Barneo J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Delpiano M. A., Acker H., Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989 May 1;486(1):79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Garber S. S., Aldrich R. W. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988 Jun 17;240(4859):1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Effect of internal fluoride and phosphate on membrane currents during intracellular dialysis of nerve cells. Nature. 1975 Oct 23;257(5528):691–693. doi: 10.1038/257691a0. [DOI] [PubMed] [Google Scholar]
- Lynch J. W., Barry P. H. Properties of transient K+ currents and underlying single K+ channels in rat olfactory receptor neurons. J Gen Physiol. 1991 May;97(5):1043–1072. doi: 10.1085/jgp.97.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. J Physiol. 1988 Feb;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso A., Almaraz L., Gonzalez C. Effects of cyanide and uncouplers on chemoreceptor activity and ATP content of the cat carotid body. Brain Res. 1989 Mar 6;481(2):250–257. doi: 10.1016/0006-8993(89)90801-9. [DOI] [PubMed] [Google Scholar]
- Obeso A., Rocher A., Fidone S., Gonzalez C. The role of dihydropyridine-sensitive Ca2+ channels in stimulus-evoked catecholamine release from chemoreceptor cells of the carotid body. Neuroscience. 1992;47(2):463–472. doi: 10.1016/0306-4522(92)90260-9. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Wagoner P. K. The inactivating K+ current in GH3 pituitary cells and its modification by chemical reagents. J Physiol. 1989 Mar;410:587–612. doi: 10.1113/jphysiol.1989.sp017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Effects of D600 on hypoxic suppression of K+ currents in isolated type I carotid body cells of the neonatal rat. FEBS Lett. 1990 Oct 1;271(1-2):37–40. doi: 10.1016/0014-5793(90)80366-q. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Almaraz L., González C. Cyclic AMP modulates differentially the release of dopamine induced by hypoxia and other stimuli and increases dopamine synthesis in the rabbit carotid body. J Neurochem. 1991 Dec;57(6):1992–2000. doi: 10.1111/j.1471-4159.1991.tb06414.x. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Almaraz L., González C. Effects of different types of stimulation on cyclic AMP content in the rabbit carotid body: functional significance. J Neurochem. 1990 Oct;55(4):1287–1293. doi: 10.1111/j.1471-4159.1990.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Rigual R., López-López J. R., Gonzalez C. Release of dopamine and chemoreceptor discharge induced by low pH and high PCO2 stimulation of the cat carotid body. J Physiol. 1991 Feb;433:519–531. doi: 10.1113/jphysiol.1991.sp018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Shaw K., Montague W., Pallot D. J. Biochemical studies on the release of catecholamines from the rat carotid body in vitro. Biochim Biophys Acta. 1989 Sep 4;1013(1):42–46. doi: 10.1016/0167-4889(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc C. K., Aldrich R. W. Gating of single non-Shaker A-type potassium channels in larval Drosophila neurons. J Gen Physiol. 1990 Jul;96(1):135–165. doi: 10.1085/jgp.96.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991 Mar;418(1-2):93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- Thorn P. J., Wang X. M., Lemos J. R. A fast, transient K+ current in neurohypophysial nerve terminals of the rat. J Physiol. 1991 Jan;432:313–326. doi: 10.1113/jphysiol.1991.sp018386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J., López-López J., González C., López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. J., Cheng G. F., Yoshizaki K., Dinger B., Fidone S. The role of cyclic AMP in chemoreception in the rabbit carotid body. Brain Res. 1991 Feb 1;540(1-2):96–104. doi: 10.1016/0006-8993(91)90495-h. [DOI] [PubMed] [Google Scholar]
- Zbicz K. L., Weight F. F. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985 Apr;53(4):1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]


