Abstract
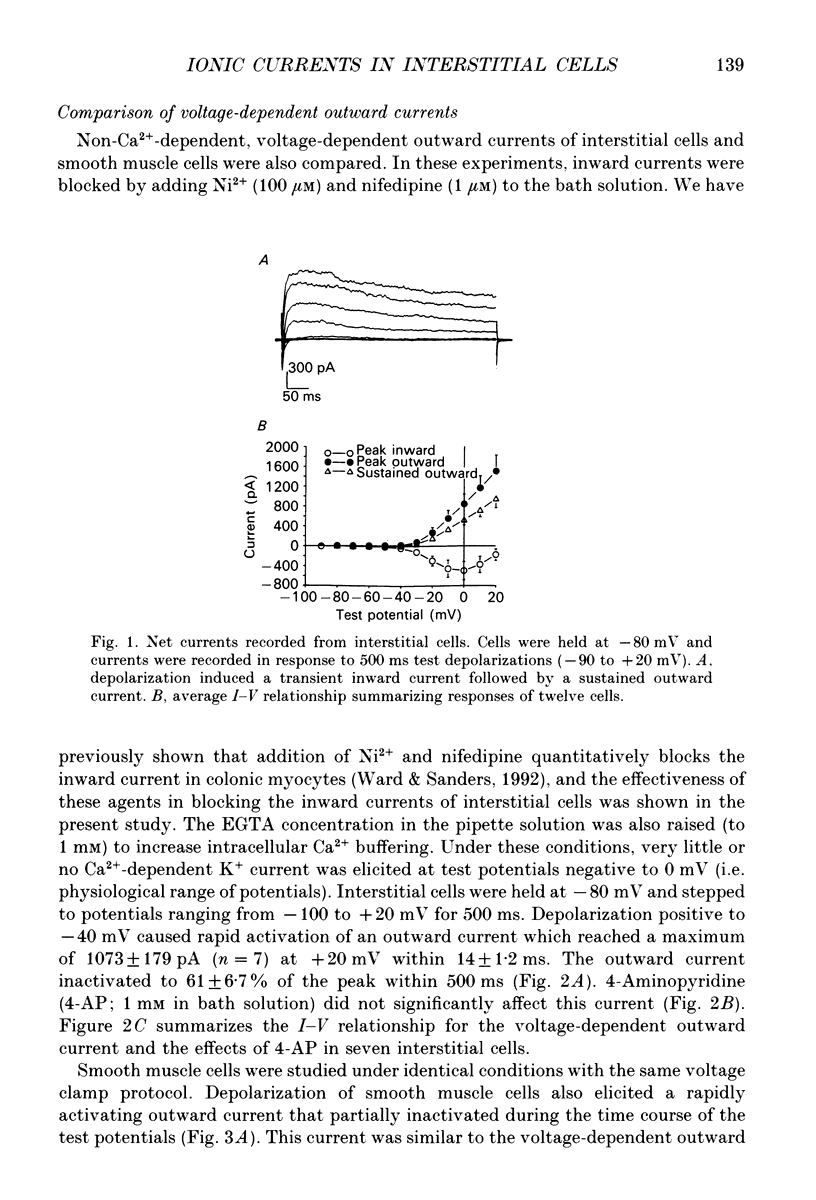
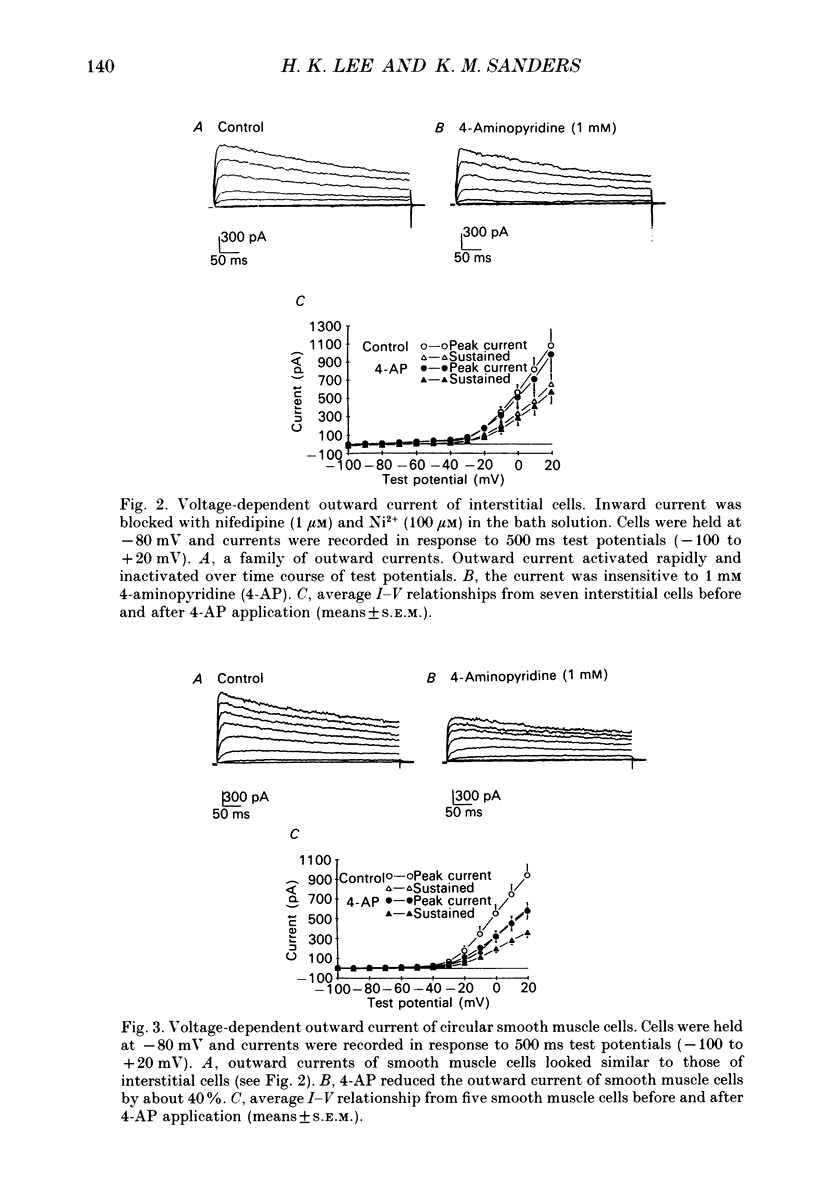
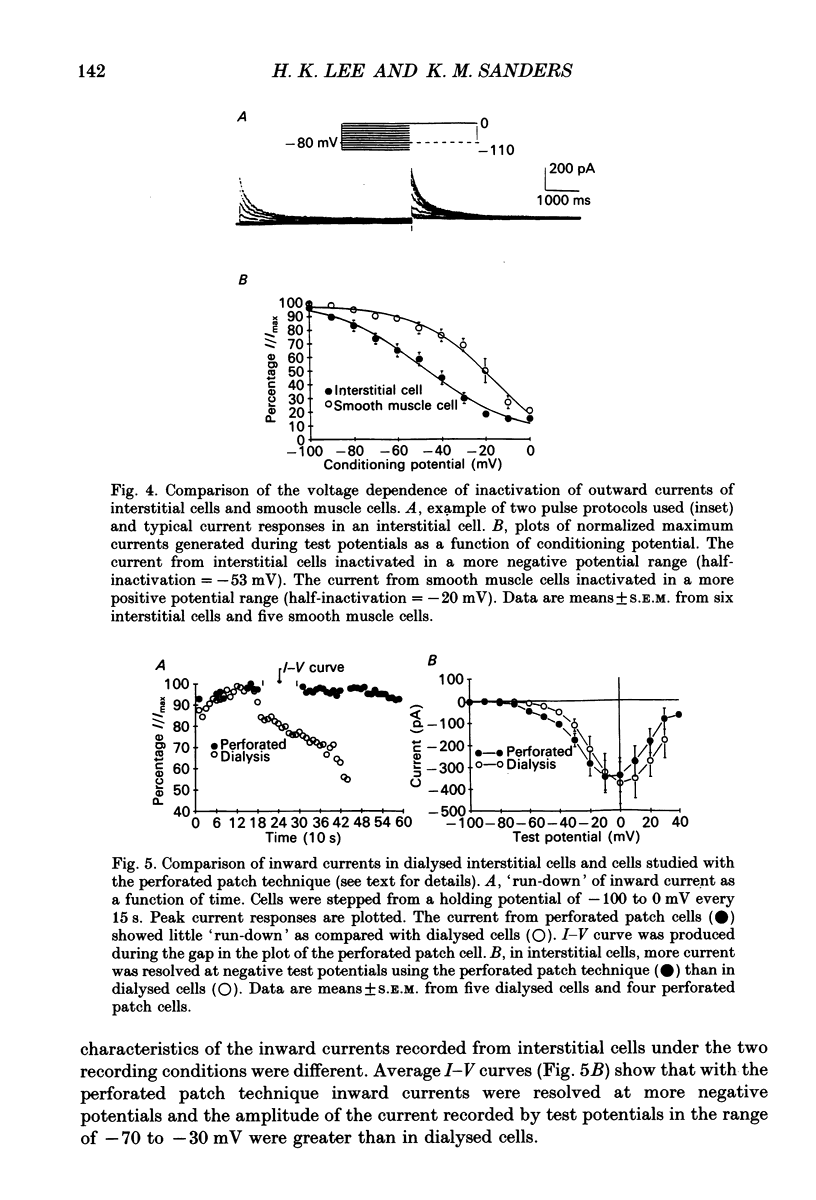
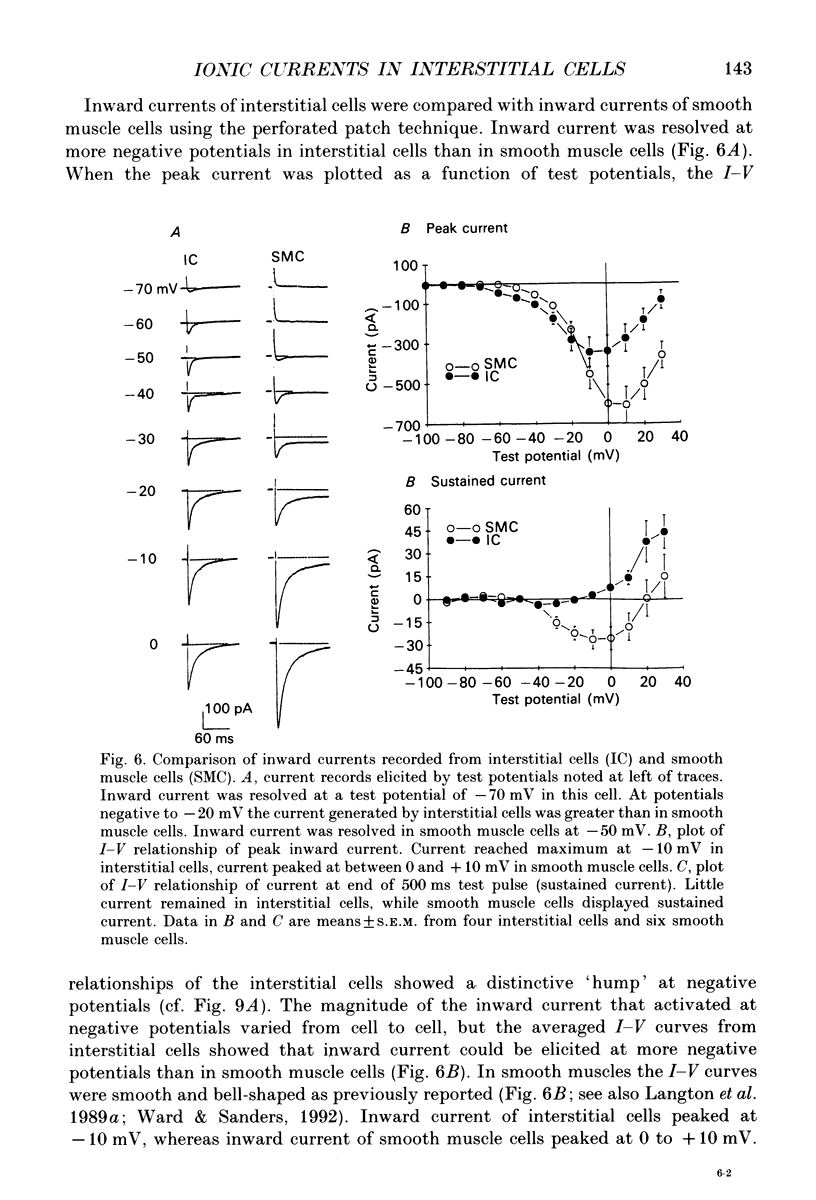
1. Voltage-dependent ionic currents of isolated interstitial cells were characterized using the whole-cell voltage clamp technique, and compared with currents recorded from circular muscle cells. Both cell types were isolated from the submucosal pacemaking region in the canine distal colon. 2. Upon depolarization, interstitial cells and smooth muscle cells generated transient inward, followed by slowly inactivating outward, currents. 3. After blocking inward current and much of the Ca(2+)-dependent outward current, interstitial cells displayed voltage-dependent outward current that rapidly activated, reached a peak, and then inactivated. This current was resistant to 4-aminopyridine(4-AP; 1 mM). Smooth muscle cells expressed a similar current but it was reduced by about 40% at a test potential of +20 mV by 4-AP (1 mM). 4. The inactivation characteristics of the voltage-dependent outward currents of interstitial cells and smooth muscle cells were compared. The outward current of interstitial cells inactivated at more negative potentials; half-inactivation occurred at -53 mV, whereas half-inactivation occurred at -20 mV in smooth muscle cells. 5. Inward currents were not strikingly different in the two cell types when dialysing pipettes were used. When the perforated patch technique (using Amphotericin-B) was used, a negatively activating inward current was observed in interstitial cells that had a resolution threshold of -70 to -60 mV. This current peaked at -10 mV. Inward currents in smooth muscle cells were resolved at test potentials positive to -50 mV and peaked at 0 to +10 mV. 6. When interstitial cells were held at -40 mV, inward current could not be resolved with test depolarization negative to -30 mV. From this holding potential, peak amplitude was reduced by 85% with test depolarizations to -10 mV. Holding smooth muscle cells at -40 mV also reduced inward current, but the peak current in these cells was reduced by only 39% at 0 mV. 7. Ni2+ partially inhibited peak inward current in interstitial cells and abolished a 'hump' in the I-V curve that occurred at negative potentials. In dialysed cells where this 'hump' was not apparent, addition of nifedipine unmasked a 'hump'. The presence of both nifedipine and Ni2+ abolished inward current. 8. A portion of the inward current in smooth muscle cells was sustained and persisted for the duration of test pulses. Very little sustained inward current was observed in interstitial cells. 9. The time course of inactivation of inward current in interstitial cells was fitted with two exponentials.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
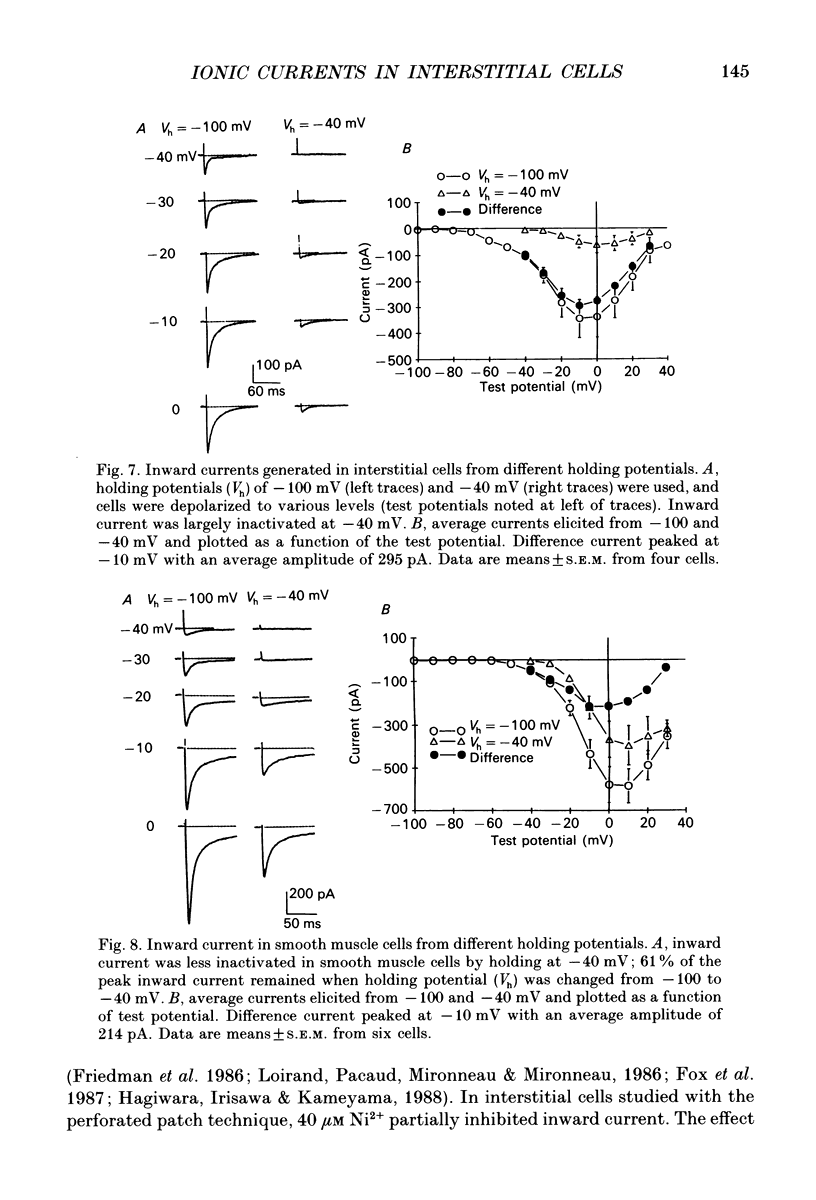
- Aaronson P. I., Bolton T. B., Lang R. J., MacKenzie I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal-calcium and high-barium solutions. J Physiol. 1988 Nov;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
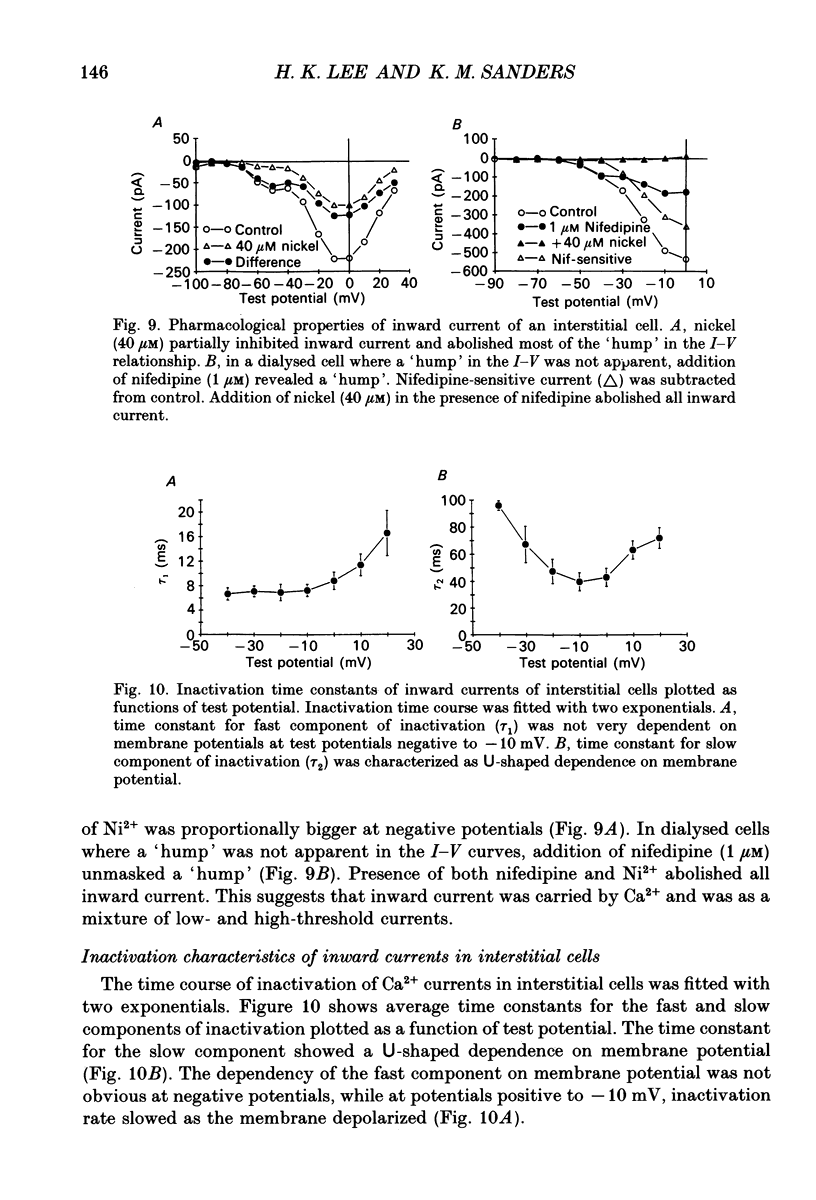
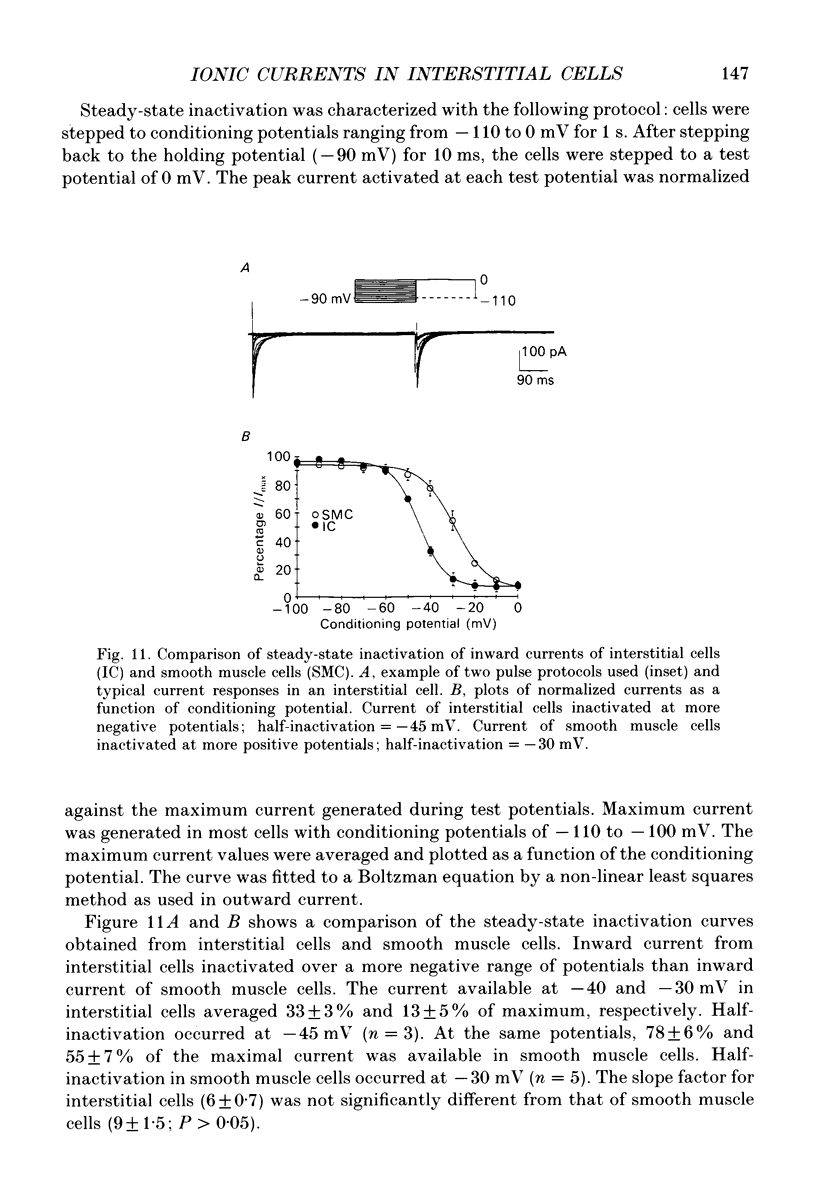
- Barajas-López C., Berezin I., Daniel E. E., Huizinga J. D. Pacemaker activity recorded in interstitial cells of Cajal of the gastrointestinal tract. Am J Physiol. 1989 Oct;257(4 Pt 1):C830–C835. doi: 10.1152/ajpcell.1989.257.4.C830. [DOI] [PubMed] [Google Scholar]
- Barajas-López C., Huizinga J. D. Different mechanisms of contraction generation in circular muscle of canine colon. Am J Physiol. 1989 Mar;256(3 Pt 1):G570–G580. doi: 10.1152/ajpgi.1989.256.3.G570. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin I., Huizinga J. D., Daniel E. E. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol. 1988 Jul 1;273(1):42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Fagni L., Feltz A. Voltage-activated calcium channels in rat Purkinje cells maintained in culture. Pflugers Arch. 1989 May;414(1):92–94. doi: 10.1007/BF00585632. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A. Inactivation of the low-threshold transient calcium current in rat sensory neurones: evidence for a dual process. J Physiol. 1986 Jul;376:341–357. doi: 10.1113/jphysiol.1986.sp016157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Christensen J., Caprilli R., Lund G. F. Electric slow waves in circular muscle of cat colon. Am J Physiol. 1969 Sep;217(3):771–776. doi: 10.1152/ajplegacy.1969.217.3.771. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. E., Suarez-Kurtz G., Kaczorowski G. J., Katz G. M., Reuben J. P. Two calcium currents in a smooth muscle cell line. Am J Physiol. 1986 Apr;250(4 Pt 2):H699–H703. doi: 10.1152/ajpheart.1986.250.4.H699. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga J. D., Chang G., Diamant N. E., El-Sharkawy T. Y. Electrophysiological basis of excitation of canine colonic circular muscle by cholinergic agents and substance P. J Pharmacol Exp Ther. 1984 Dec;231(3):692–699. [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Burke E. P., Sanders K. M. Participation of Ca currents in colonic electrical activity. Am J Physiol. 1989 Sep;257(3 Pt 1):C451–C460. doi: 10.1152/ajpcell.1989.257.3.C451. [DOI] [PubMed] [Google Scholar]
- Langton P., Ward S. M., Carl A., Norell M. A., Sanders K. M. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Mironneau C., Mironneau J. Evidence for two distinct calcium channels in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1986 Nov;407(5):566–568. doi: 10.1007/BF00657519. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Stevens R. J., Blondfield D. P., Publicover N. G., Sanders K. M. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol. 1991 May;260(5 Pt 1):C917–C925. doi: 10.1152/ajpcell.1991.260.5.C917. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Sanders K. M. Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annu Rev Physiol. 1992;54:439–453. doi: 10.1146/annurev.ph.54.030192.002255. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Smith T. K. Enteric neural regulation of slow waves in circular muscle of the canine proximal colon. J Physiol. 1986 Aug;377:297–313. doi: 10.1113/jphysiol.1986.sp016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M., Smith T. K. Motoneurones of the submucous plexus regulate electrical activity of the circular muscle of canine proximal colon. J Physiol. 1986 Nov;380:293–310. doi: 10.1113/jphysiol.1986.sp016286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., McCleskey E. W. T-type calcium channels: heterogeneous expression in rat sensory neurons and selective modulation by phorbol esters. J Neurosci. 1990 Mar;10(3):947–951. doi: 10.1523/JNEUROSCI.10-03-00947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987 Feb;252(2 Pt 1):C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Lightowler S., Leresche N., Jassik-Gerschenfeld D., Pollard C. E., Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991 Sep;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Prosser C. L., Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am J Physiol. 1986 Mar;250(3 Pt 1):G287–G294. doi: 10.1152/ajpgi.1986.250.3.G287. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Ward S. M., Sanders K. M. Participation of fast-activating, voltage-dependent K currents in electrical slow waves of colonic circular muscle. Am J Physiol. 1992 Jul;263(1 Pt 1):C226–C236. doi: 10.1152/ajpcell.1992.263.1.C226. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Tseng G. N., Boyden P. A. Multiple types of Ca2+ currents in single canine Purkinje cells. Circ Res. 1989 Dec;65(6):1735–1750. doi: 10.1161/01.res.65.6.1735. [DOI] [PubMed] [Google Scholar]
- Vivaudou M. B., Singer J. J., Walsh J. V., Jr Multiple types of Ca2+ channels in visceral smooth muscle cells. Pflugers Arch. 1991 Mar;418(1-2):144–152. doi: 10.1007/BF00370463. [DOI] [PubMed] [Google Scholar]
- Vogalis F., Publicover N. G., Hume J. R., Sanders K. M. Relationship between calcium current and cytosolic calcium in canine gastric smooth muscle cells. Am J Physiol. 1991 May;260(5 Pt 1):C1012–C1018. doi: 10.1152/ajpcell.1991.260.5.C1012. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Burke E. P., Sanders K. M. Use of rhodamine 123 to label and lesion interstitial cells of Cajal in canine colonic circular muscle. Anat Embryol (Berl) 1990;182(3):215–224. doi: 10.1007/BF00185515. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Thornbury K. D., Westfall D. P., Sanders K. M. Nonadrenergic, noncholinergic inhibition and rebound excitation in canine colon depend on nitric oxide. Am J Physiol. 1992 Feb;262(2 Pt 1):G237–G243. doi: 10.1152/ajpgi.1992.262.2.G237. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Keller R. G., Sanders K. M. Structure and organization of electrical activity of canine distal colon. Am J Physiol. 1991 May;260(5 Pt 1):G724–G735. doi: 10.1152/ajpgi.1991.260.5.G724. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Sanders K. M. Pacemaker activity in septal structures of canine colonic circular muscle. Am J Physiol. 1990 Aug;259(2 Pt 1):G264–G273. doi: 10.1152/ajpgi.1990.259.2.G264. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Sanders K. M. Upstroke component of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant calcium current. J Physiol. 1992 Sep;455:321–337. doi: 10.1113/jphysiol.1992.sp019304. [DOI] [PMC free article] [PubMed] [Google Scholar]


