Abstract
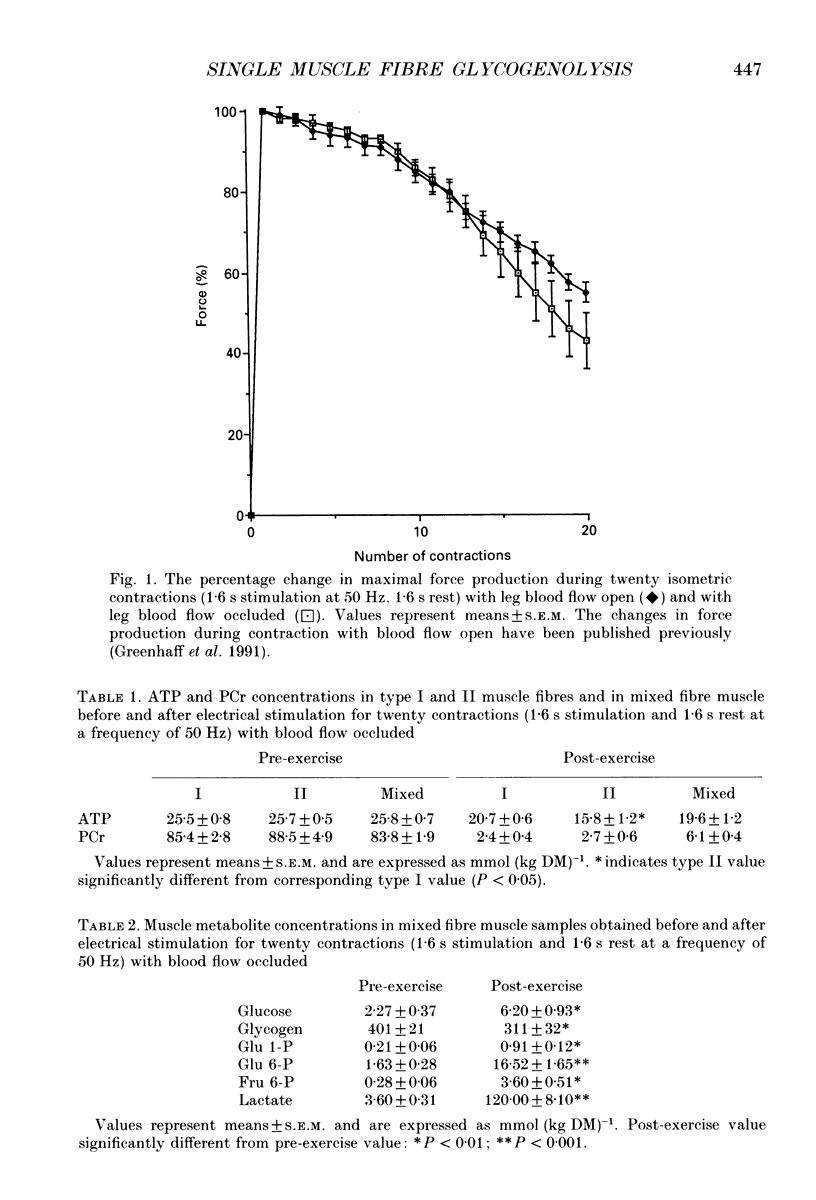
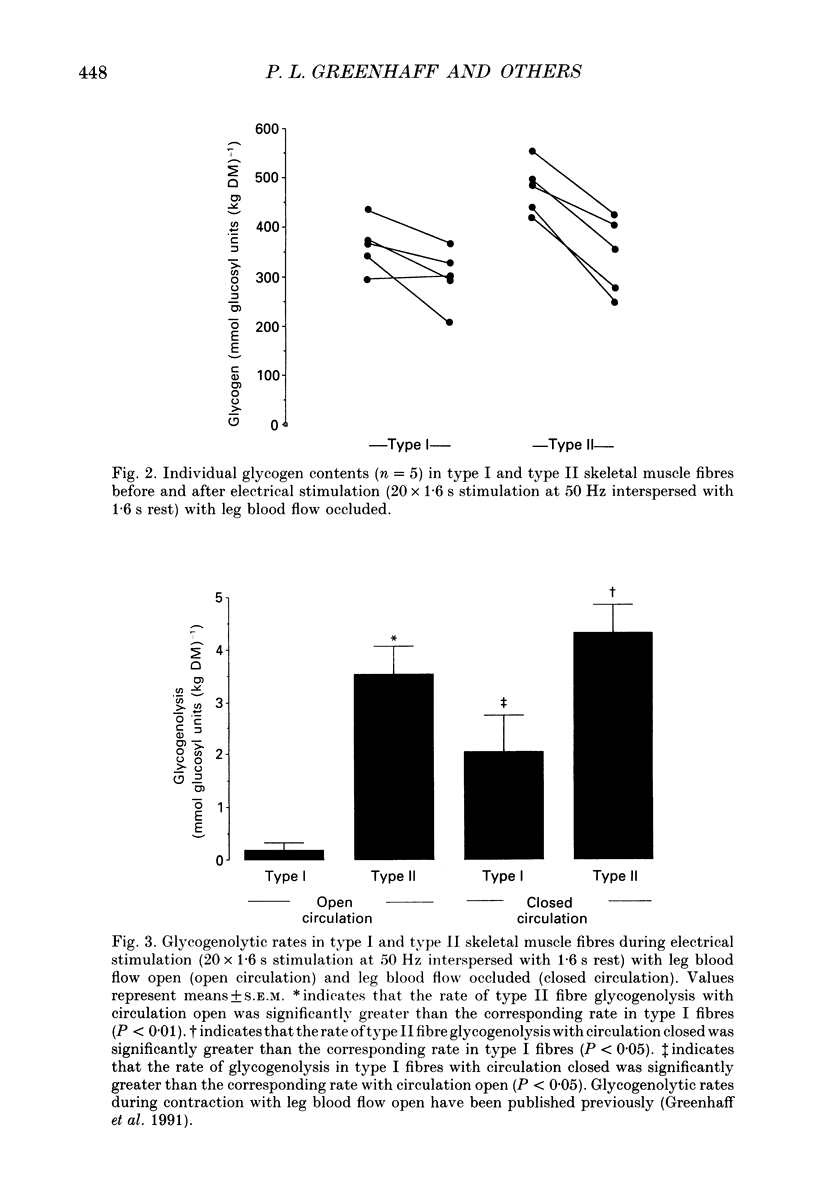
1. Glycogenolysis in type I and II muscle fibres was investigated in five healthy volunteers during electrical stimulation of the quadriceps muscle group with blood flow occluded. 2. The quadriceps femoris muscles were stimulated intermittently (1.6 s stimulation, 1.6 s rest) at a frequency of 50 Hz for 64 s and isometric contraction force was recorded. Muscle biopsies were obtained at rest prior to and immediately after stimulation. Single muscle fibres were dissected free and were identified as type I and II fibres. ATP, phosphocreatine (PCr) and glycogen contents were measured luminometrically and enzymatically in single fibres and mixed fibre muscle. 3. Electrical stimulation resulted in a marked decline in contraction force and near total depletion of PCr in both fibre types. The ATP turnover rate (P < 0.05) and the magnitude of the decline in ATP (P < 0.05) were greater in type II fibres. Prior to stimulation the muscle glycogen content was 32% higher in type II fibres compared with type I fibres (P < 0.01). During stimulation the rate of glycogenolysis in type II fibres (4.32 +/- 0.54 mmol (kg dry matter (DM)-1 s-1 was twofold greater than the rate in type I fibres (2.05 +/- 0.70 mmol (kg DM)-1 s-1, P < 0.05). 4. The data suggest that the relatively higher rate of glycogenolysis observed in type I fibres during intermittent electrical stimulation with occluded circulation (2.05 +/- 0.70 mmol (kg DM)-1 s-1), when compared with the corresponding rate recorded during intense contraction with circulation intact (0.18 +/- 0.14 mmol (kg DM)-1 s-1, P < 0.05), may result from an accelerated ATP turnover rate in this fibre type increasing the cellular concentrations of free AMP and inosine 5'-monophosphate (IMP), which are known activators of glycogen phosphorylase. 5. The similarity in the rate of type II fibre glycogenolysis during contraction with circulatory occlusion (4.32 +/- 0.54 mmol (kg DM)-1 s-1), when compared with the corresponding rate recorded during non-occluded circulation (3.54 +/- 0.53 mmol (kg DM)-1 s-1, P > 0.05), is in agreement with the suggestion that glycogenolysis in this fibre type is already occurring at a near-maximal rate with circulation intact.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A., McCubbrey D. A. Power outputs of slow and fast skeletal muscles of mice. J Appl Physiol (1985) 1990 Mar;68(3):1282–1285. doi: 10.1152/jappl.1990.68.3.1282. [DOI] [PubMed] [Google Scholar]
- CORI G. T., ILLINGWORTH B. The effect of epinephrine and other glycogenolytic agents on the phosphorylase A content of muscle. Biochim Biophys Acta. 1956 Jul;21(1):105–110. doi: 10.1016/0006-3002(56)90099-3. [DOI] [PubMed] [Google Scholar]
- Chasiotis D., Sahlin K., Hultman E. Regulation of glycogenolysis in human muscle in response to epinephrine infusion. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jan;54(1):45–50. doi: 10.1152/jappl.1983.54.1.45. [DOI] [PubMed] [Google Scholar]
- Chasiotis D. The regulation of glycogen phosphorylase and glycogen breakdown in human skeletal muscle. Acta Physiol Scand Suppl. 1983;518:1–68. [PubMed] [Google Scholar]
- Conlee R. K., McLane J. A., Rennie M. J., Winder W. W., Holloszy J. O. Reversal of phosphorylase activation in muscle despite continued contractile activity. Am J Physiol. 1979 Nov;237(5):R291–R296. doi: 10.1152/ajpregu.1979.237.5.R291. [DOI] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E., CORICF The effect of contraction and of epinephrine on the phosphorylase activity of frog sartorius muscle. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1191–1199. doi: 10.1073/pnas.48.7.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley G. A., Terjung R. L. Influence of aerobic metabolism on IMP accumulation in fast-twitch muscle. Am J Physiol. 1985 Jan;248(1 Pt 1):C37–C42. doi: 10.1152/ajpcell.1985.248.1.C37. [DOI] [PubMed] [Google Scholar]
- Essén B., Jansson E., Henriksson J., Taylor A. W., Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975 Oct;95(2):153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Greenhaff P. L., Ren J. M., Söderlund K., Hultman E. Energy metabolism in single human muscle fibers during contraction without and with epinephrine infusion. Am J Physiol. 1991 May;260(5 Pt 1):E713–E718. doi: 10.1152/ajpendo.1991.260.5.E713. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Essén B., Hultman E. Glycogen phosphorylase activity in biopsy samples and single muscle fibres of musculus quadriceps femoris of man at rest. Scand J Clin Lab Invest. 1976 Oct;36(6):521–526. doi: 10.1080/00365517609054473. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Hintz C. S., Chi M. M., Fell R. D., Ivy J. L., Kaiser K. K., Lowry C. V., Lowry O. H. Metabolite changes in individual rat muscle fibers during stimulation. Am J Physiol. 1982 Mar;242(3):C218–C228. doi: 10.1152/ajpcell.1982.242.3.C218. [DOI] [PubMed] [Google Scholar]
- Hultman E., Sjöholm H. Energy metabolism and contraction force of human skeletal muscle in situ during electrical stimulation. J Physiol. 1983 Dec;345:525–532. doi: 10.1113/jphysiol.1983.sp014994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman E., Sjöholm H., Jäderholm-Ek I., Krynicki J. Evaluation of methods for electrical stimulation of human skeletal muscle in situ. Pflugers Arch. 1983 Jul;398(2):139–141. doi: 10.1007/BF00581062. [DOI] [PubMed] [Google Scholar]
- Jansson E., Dudley G. A., Norman B., Tesch P. A. ATP and IMP in single human muscle fibres after high intensity exercise. Clin Physiol. 1987 Aug;7(4):337–345. doi: 10.1111/j.1475-097x.1987.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Katz A., Sahlin K., Henriksson J. Muscle ATP turnover rate during isometric contraction in humans. J Appl Physiol (1985) 1986 Jun;60(6):1839–1842. doi: 10.1152/jappl.1986.60.6.1839. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., SCHULZ D. W., PASSONNEAU J. V. EFFECTS OF ADENYLIC ACID ON THE KINETICS OF MUSCLE PHOSPHORYLASE A. J Biol Chem. 1964 Jun;239:1947–1953. [PubMed] [Google Scholar]
- Meyer R. A., Dudley G. A., Terjung R. L. Ammonia and IMP in different skeletal muscle fibers after exercise in rats. J Appl Physiol Respir Environ Exerc Physiol. 1980 Dec;49(6):1037–1041. doi: 10.1152/jappl.1980.49.6.1037. [DOI] [PubMed] [Google Scholar]
- Ren J. M., Hultman E. Regulation of glycogenolysis in human skeletal muscle. J Appl Physiol (1985) 1989 Dec;67(6):2243–2248. doi: 10.1152/jappl.1989.67.6.2243. [DOI] [PubMed] [Google Scholar]
- Ren J. M., Hultman E. Regulation of phosphorylase a activity in human skeletal muscle. J Appl Physiol (1985) 1990 Sep;69(3):919–923. doi: 10.1152/jappl.1990.69.3.919. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Fell R. D., Ivy J. L., Holloszy J. O. Adrenaline reactivation of muscle phosphorylase after deactivation during phasic contractile activity. Biosci Rep. 1982 May;2(5):323–331. doi: 10.1007/BF01115118. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Broberg S., Ren J. M. Formation of inosine monophosphate (IMP) in human skeletal muscle during incremental dynamic exercise. Acta Physiol Scand. 1989 Jun;136(2):193–198. doi: 10.1111/j.1748-1716.1989.tb08652.x. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Gorski J., Edström L. Influence of ATP turnover and metabolite changes on IMP formation and glycolysis in rat skeletal muscle. Am J Physiol. 1990 Sep;259(3 Pt 1):C409–C412. doi: 10.1152/ajpcell.1990.259.3.C409. [DOI] [PubMed] [Google Scholar]
- Saltin B., Henriksson J., Nygaard E., Andersen P., Jansson E. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann N Y Acad Sci. 1977;301:3–29. doi: 10.1111/j.1749-6632.1977.tb38182.x. [DOI] [PubMed] [Google Scholar]
- Söderlund K., Greenhaff P. L., Hultman E. Energy metabolism in type I and type II human muscle fibres during short term electrical stimulation at different frequencies. Acta Physiol Scand. 1992 Jan;144(1):15–22. doi: 10.1111/j.1748-1716.1992.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Tullson P. C., Whitlock D. M., Terjung R. L. Adenine nucleotide degradation in slow-twitch red muscle. Am J Physiol. 1990 Feb;258(2 Pt 1):C258–C265. doi: 10.1152/ajpcell.1990.258.2.C258. [DOI] [PubMed] [Google Scholar]


