Abstract
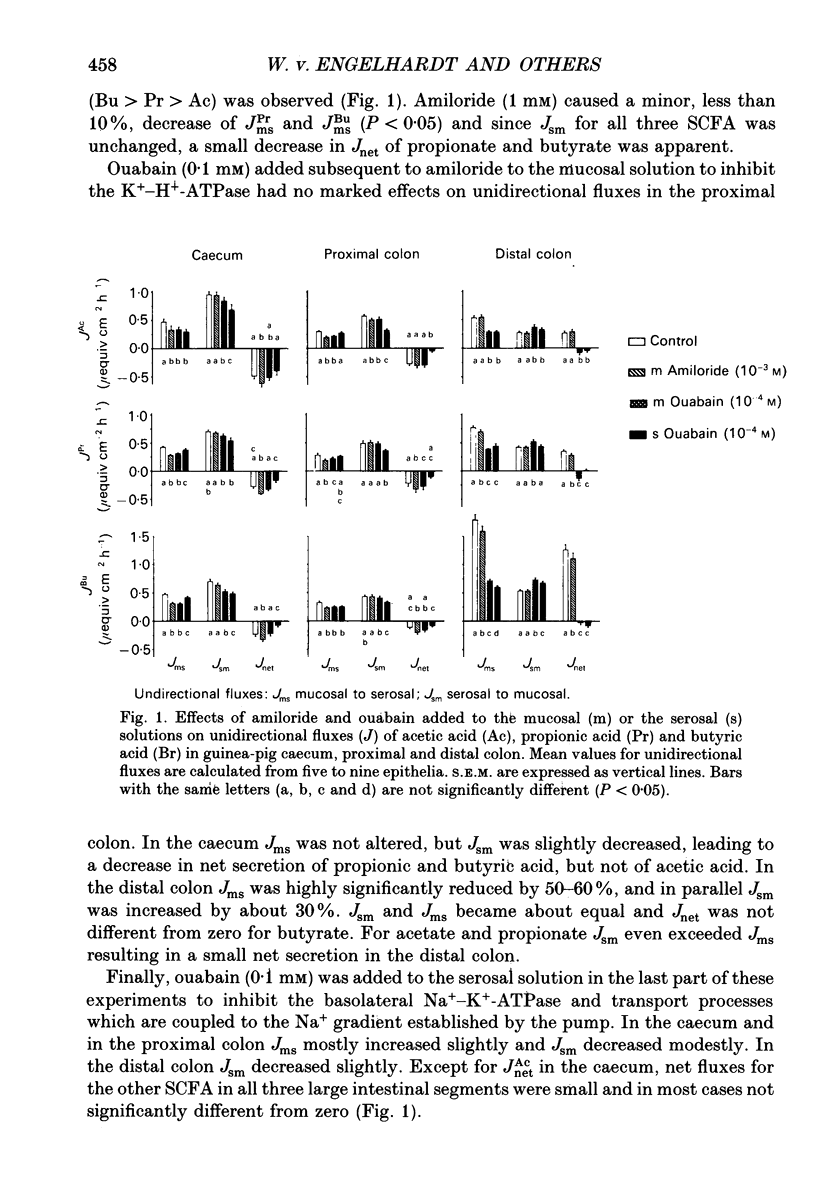
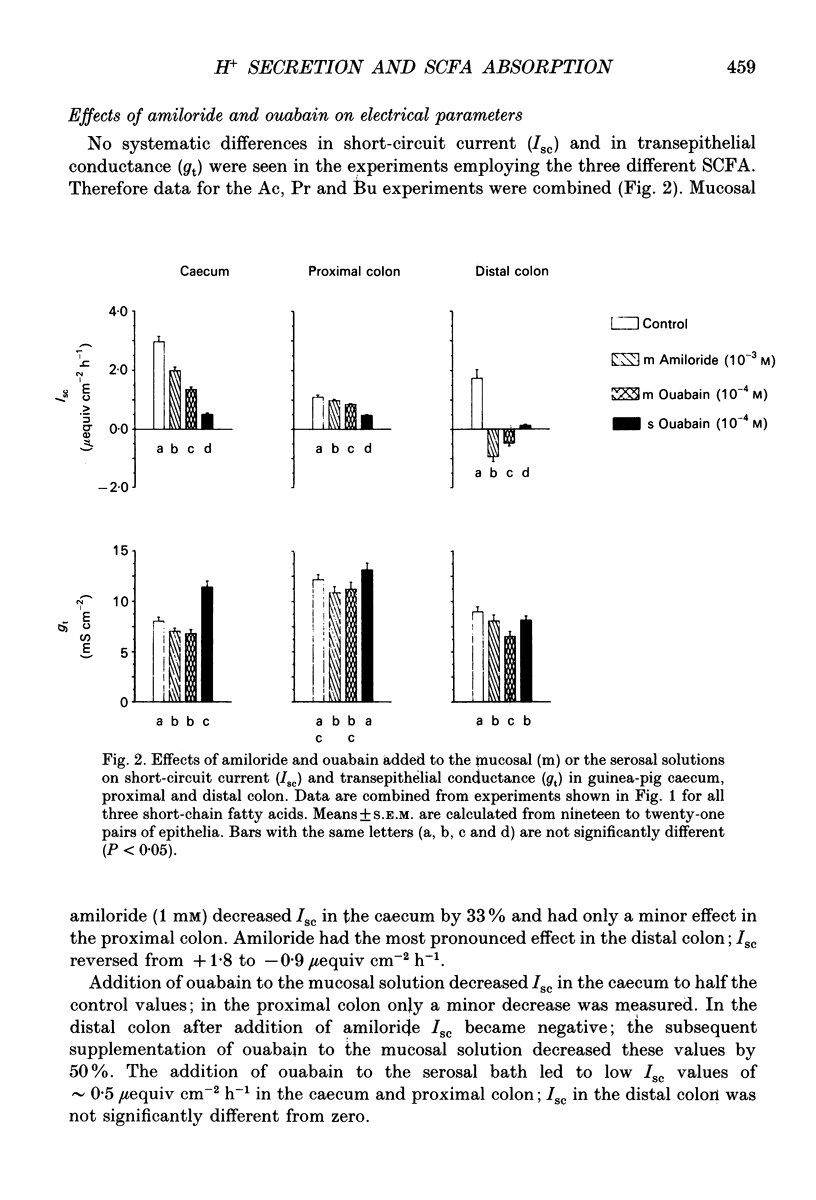
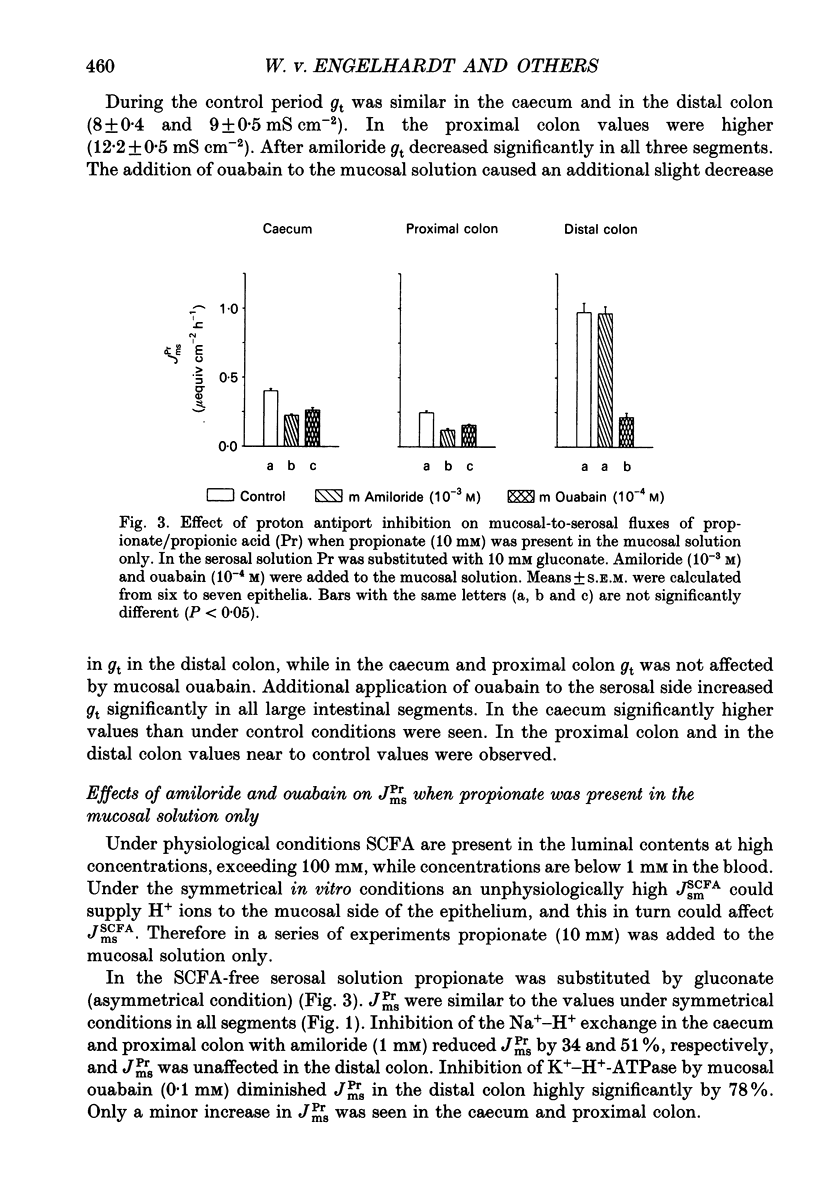
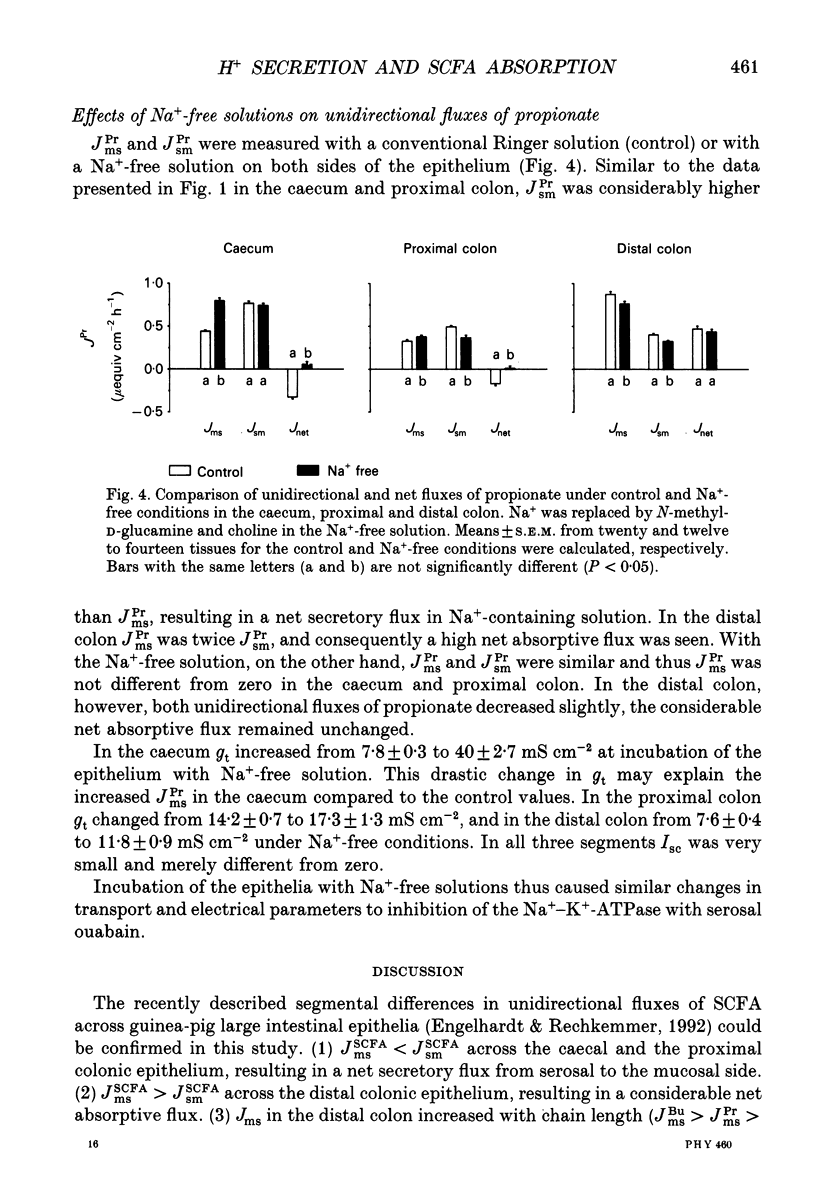
1. Effects of H+ secreting mechanisms on unidirectional passage of the short-chain fatty acids (SCFA) acetate, propionate and n-butyrate across isolated guinea-pig caecum, proximal and distal colon were studied under short-circuit current conditions in Ussing chamber isotope flux experiments. 2. In the caecum and the proximal colon the serosal-to-mucosal fluxes (Jsm) were higher than the mucosal-to-serosal fluxes (Jms). Thus a net secretion of SCFA was present in the caecum and proximal colon. The higher Jsm appears to be coupled to the Na+ gradient established by the basolateral membrane Na(+)-K(+)-ATPase, whereas Jms is related to the operation of an apical membrane Na(+)-H+ exchanger. Inhibition of Na(+)-H+ exchange by amiloride (1 mM) added to the mucosal solution decreased Jms of SCFA in caecum and in proximal colon, but had no major effect in distal colon. 3. In distal colon Jms exceeds Jsm and thus a net absorption of SCFA was observed. Jms is Na+ independent and coupled to the activity of the apical membrane K(+)-H(+)-ATPase. Inhibition of the K(+)-H(+)-ATPase by addition of ouabain (0.1 mM) to the mucosal solution diminished Jms in the distal colon; in the caecum and proximal colon ouabain had no effect on Jms. 4. Neither amiloride nor ouabain caused major changes in Jsm in any of the large intestinal segments. 5. In conclusion, absorption of SCFA, e.g. Jms, in all large intestinal segments is related to the presence and activity of H+ secreting systems located in the apical membrane of colonocytes. In the caecum and proximal colon the predominant system appears to be Na(+)-H+ exchange, and in the distal colon K(+)-H(+)-ATPase. Supply of H+ ions allows protonation of SCFA anions and subsequent permeation by non-ionic diffusion across the apical membrane. These mechanisms account for 35, 40-50 and 60-80% of SCFA transport in the caecum, the proximal and the distal colon of guinea-pig, respectively. The nature of the Na(+)-dependent secretory pathway in the caecum and proximal colon remains to be determined.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder H. J., Mehta P. Characterization of butyrate-dependent electroneutral Na-Cl absorption in the rat distal colon. Pflugers Arch. 1990 Dec;417(4):365–369. doi: 10.1007/BF00370654. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989 Apr;96(4):989–996. doi: 10.1016/0016-5085(89)91614-4. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Stange G., Murer H., Stieger B., Hauri H. P. Sodium-proton exchange in colon brush-border membranes. Am J Physiol. 1986 Sep;251(3 Pt 1):G382–G390. doi: 10.1152/ajpgi.1986.251.3.G382. [DOI] [PubMed] [Google Scholar]
- Foster E. S., Dudeja P. K., Brasitus T. A. Contribution of Cl(-)-OH- exchange to electroneutral NaCl absorption in rat distal colon. Am J Physiol. 1990 Feb;258(2 Pt 1):G261–G267. doi: 10.1152/ajpgi.1990.258.2.G261. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Koch M. J., Schultz S. G. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol. 1976;27(3):297–316. doi: 10.1007/BF01869142. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Frizzell R. A. Active K transport across rabbit distal colon: relation to Na absorption and Cl secretion. Am J Physiol. 1986 Aug;251(2 Pt 1):C252–C267. doi: 10.1152/ajpcell.1986.251.2.C252. [DOI] [PubMed] [Google Scholar]
- Harig J. M., Soergel K. H., Barry J. A., Ramaswamy K. Transport of propionate by human ileal brush-border membrane vesicles. Am J Physiol. 1991 May;260(5 Pt 1):G776–G782. doi: 10.1152/ajpgi.1991.260.5.G776. [DOI] [PubMed] [Google Scholar]
- Hatch M. Short-chain fatty acid transport and its effects on ion transport by rabbit cecum. Am J Physiol. 1987 Aug;253(2 Pt 1):G171–G178. doi: 10.1152/ajpgi.1987.253.2.G171. [DOI] [PubMed] [Google Scholar]
- Kuwahara A., Radowicz-Cooke H. J. Epithelial transport in guinea-pig proximal colon: influence of enteric neurones. J Physiol. 1988 Jan;395:271–284. doi: 10.1113/jphysiol.1988.sp016918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano L., Reale E., Rechkemmer G., von Engelhardt W. Structure of zonulae occludentes and the permeability of the epithelium to short-chain fatty acids in the proximal and the distal colon of guinea pig. J Membr Biol. 1984;82(2):145–156. doi: 10.1007/BF01868939. [DOI] [PubMed] [Google Scholar]
- Mascolo N., Rajendran V. M., Binder H. J. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991 Aug;101(2):331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin M. L., McBride D. E., Perrone R. D. Secondary hyperaldosteronism stimulates acidification in rat distal colon. Pflugers Arch. 1990 Aug;416(6):639–645. doi: 10.1007/BF00370608. [DOI] [PubMed] [Google Scholar]
- Perrone R. D., McBride D. E. Aldosterone and PCO2 enhance rubidium absorption in rat distal colon. Am J Physiol. 1988 Jun;254(6 Pt 1):G898–G906. doi: 10.1152/ajpgi.1988.254.6.G898. [DOI] [PubMed] [Google Scholar]
- Rajendran V. M., Binder H. J. Characterization of Na-H exchange in apical membrane vesicles of rat colon. J Biol Chem. 1990 May 25;265(15):8408–8414. [PubMed] [Google Scholar]
- Rechkemmer G., Rönnau K., von Engelhardt W. Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp Biochem Physiol A Comp Physiol. 1988;90(4):563–568. doi: 10.1016/0300-9629(88)90668-8. [DOI] [PubMed] [Google Scholar]
- Rönnau K., Guth D., von Engelhardt W. Absorption of dissociated and undissociated short-chain fatty acids across the colonic epithelium of guinea-pig. Q J Exp Physiol. 1989 Jul;74(4):511–519. doi: 10.1113/expphysiol.1989.sp003298. [DOI] [PubMed] [Google Scholar]
- Sellin J. H., DeSoignie R. Short-chain fatty acid absorption in rabbit colon in vitro. Gastroenterology. 1990 Sep;99(3):676–683. doi: 10.1016/0016-5085(90)90954-y. [DOI] [PubMed] [Google Scholar]
- Sellin J. H., Oyarzabal H., Cragoe E. J. Electrogenic sodium absorption in rabbit cecum in vitro. J Clin Invest. 1988 Apr;81(4):1275–1283. doi: 10.1172/JCI113445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Kaneko K. Acid secretion in isolated guinea pig colon. Am J Physiol. 1987 Aug;253(2 Pt 1):G155–G164. doi: 10.1152/ajpgi.1987.253.2.G155. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Kaneko K. Ouabain-sensitive H+-K+ exchange mechanism in the apical membrane of guinea pig colon. Am J Physiol. 1989 Jun;256(6 Pt 1):G979–G988. doi: 10.1152/ajpgi.1989.256.6.G979. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Suzuki T., Suzuki Y. Ouabain-sensitive K(+)-ATPase in epithelial cells from guinea pig distal colon. Am J Physiol. 1990 Apr;258(4 Pt 1):G506–G511. doi: 10.1152/ajpgi.1990.258.4.G506. [DOI] [PubMed] [Google Scholar]
- Watson A. J., Brennan E. A., Farthing M. J., Fairclough P. D. Acetate uptake by intestinal brush border membrane vesicles. Gut. 1991 Apr;32(4):383–385. doi: 10.1136/gut.32.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt W., Rechkemmer G. Segmental differences of short-chain fatty acid transport across guinea-pig large intestine. Exp Physiol. 1992 May;77(3):491–499. doi: 10.1113/expphysiol.1992.sp003609. [DOI] [PubMed] [Google Scholar]


