Abstract
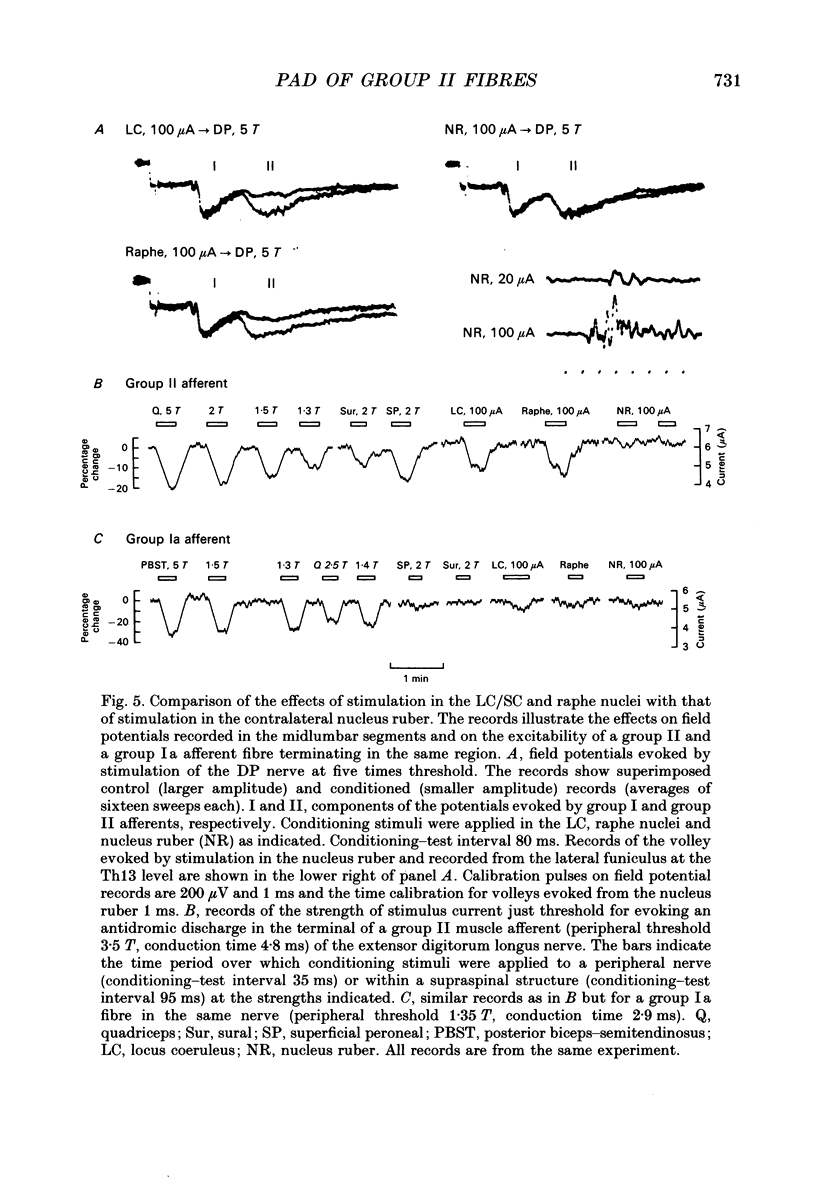
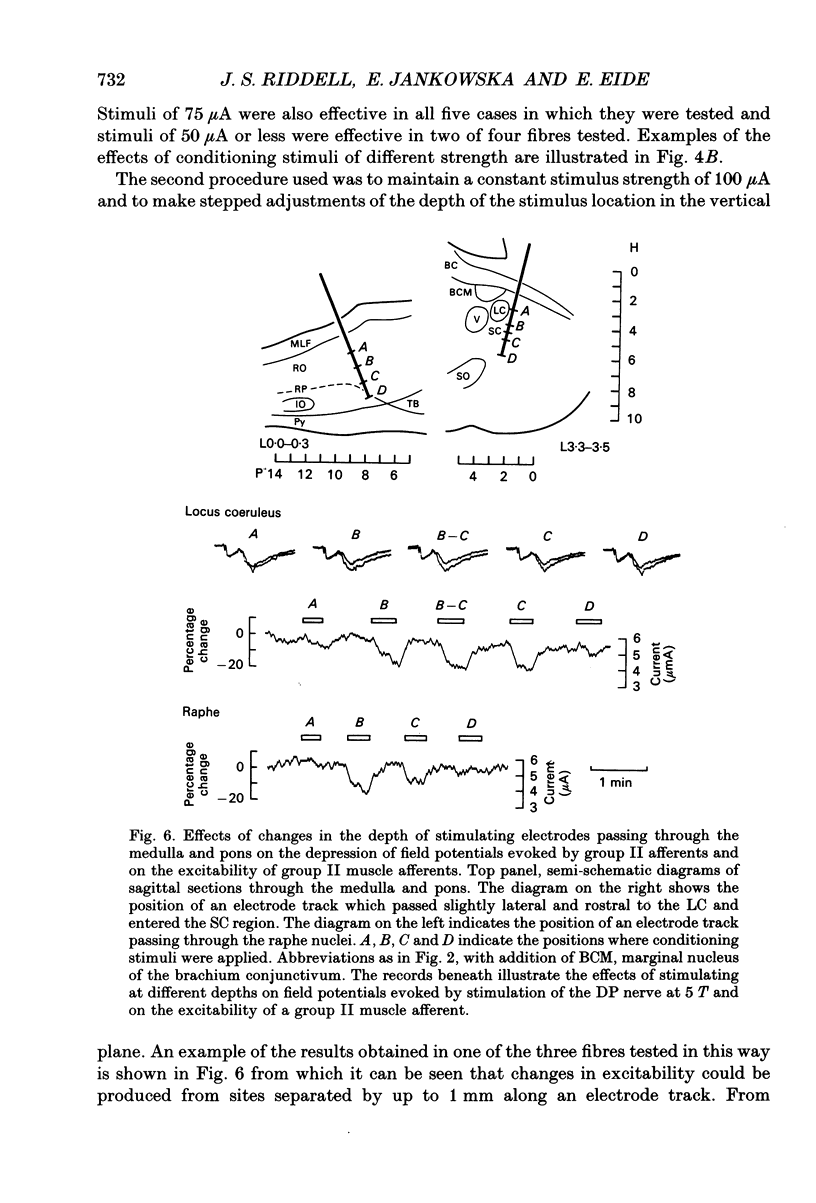
1. Electrical stimuli applied in the locus coeruleus/subcoeruleus (LC/SC) and raphe nuclei produce a profound depression of transmission in reflex pathways from group II muscle afferents. The present experiments were performed to determine whether presynaptic inhibitory mechanisms contribute to these effects. 2. Changes in the excitability of afferent terminals to electrical stimuli have been used as an indication of primary afferent depolarization (PAD) produced by conditioning stimuli applied within the LC/SC and raphe nuclei and, for comparison, in the nucleus ruber. Group II afferents originating from ankle flexor muscles and terminating in the midlumbar segments were used for testing. 3. Clear changes in excitability were observed in fourteen of nineteen group II fibres in which the effects of conditioning stimuli applied in the LC/SC were tested and in twelve of seventeen fibres in which the effects of stimuli applied within the raphe nuclei were tested. By comparison, only one of the twelve fibres tested with conditioning stimuli applied to the nucleus ruber was found to be influenced. These effects matched those of the same conditioning stimuli on field potentials evoked by group II afferents at the location at which the terminals of group II fibres were stimulated. 4. Stimuli applied in the LC/SC and in the raphe nuclei both produced a mean decrease in threshold stimulus current of 19%. These effects are comparable to those produced by the most effective volleys in peripheral afferent which, in the same fibres, produced a mean decrease in threshold stimulus current of 24%. 5. In all cases (twelve) in which the effects of stimuli applied in the LC/SC and raphe nuclei were tested on the same group II fibre, either both or neither were found to be effective. This strengthens previous indications that some populations of neurones might be activated by stimuli applied in each of these regions of the brain. 6. In contrast to group II afferents, group Ia afferents investigated in the same experiments were only exceptionally affected. Of seven fibres tested with stimuli applied in the LC/SC, six with stimuli applied in the raphe nuclei and seven with stimuli applied in the nucleus ruber, only one fibre showed any clear change in threshold and this was a single fibre which was similarly affected by stimuli in all three sites. 7. It is concluded that presynaptic inhibitory mechanisms contribute to the depression of transmission in spinal reflex pathways from group II muscle afferents produced by stimulation in the LC/SC and raphe nuclei.
Full text
PDF

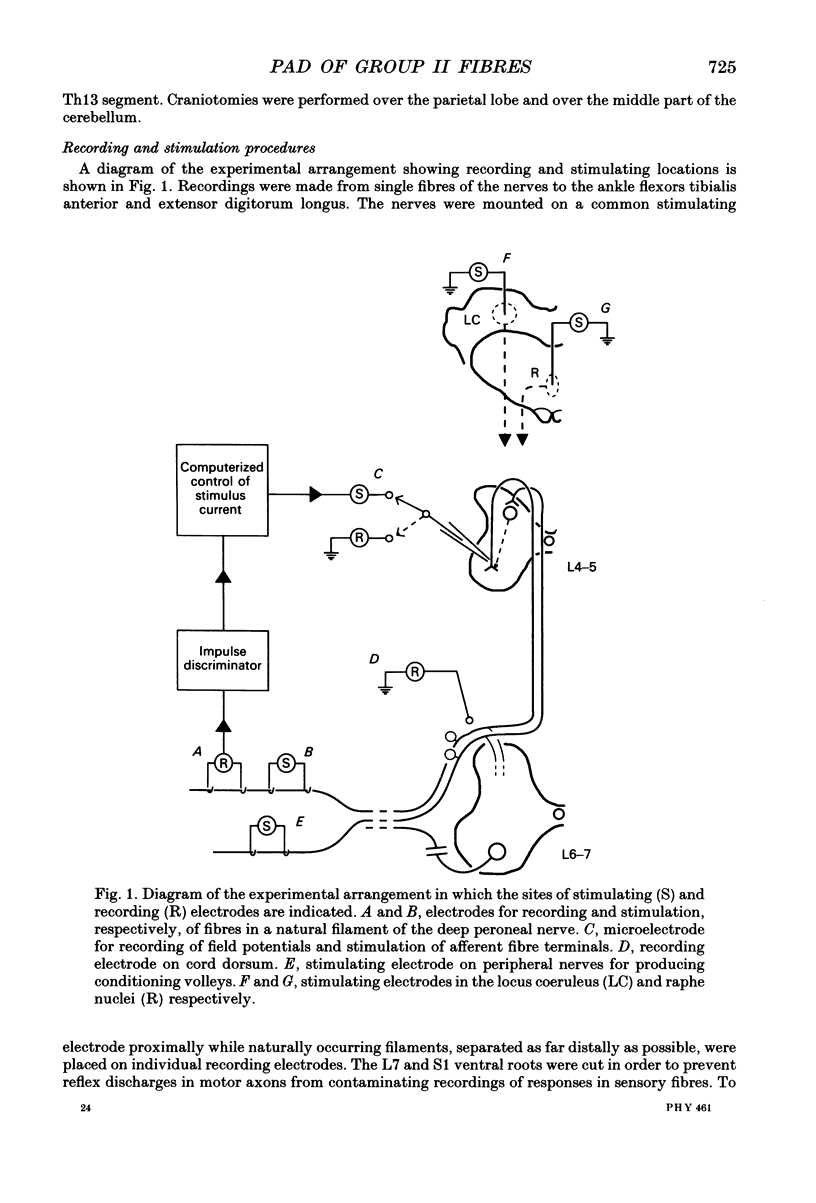
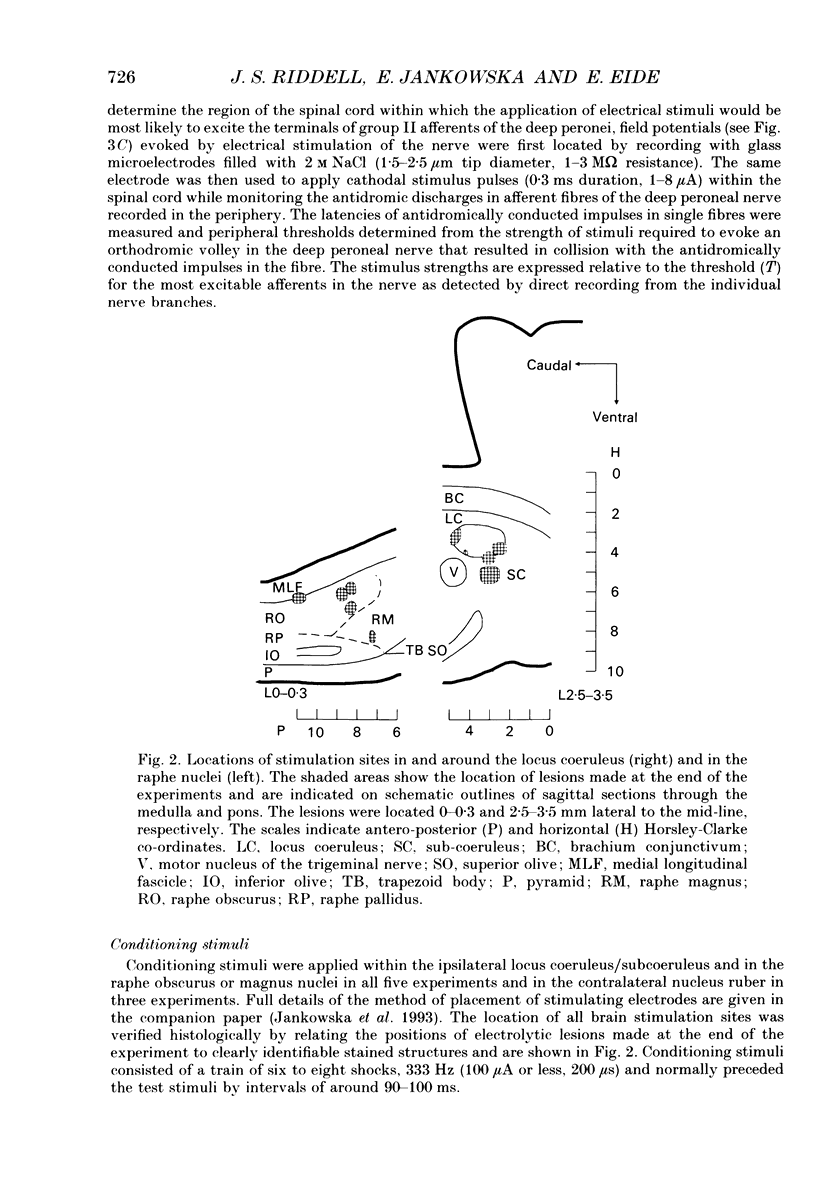

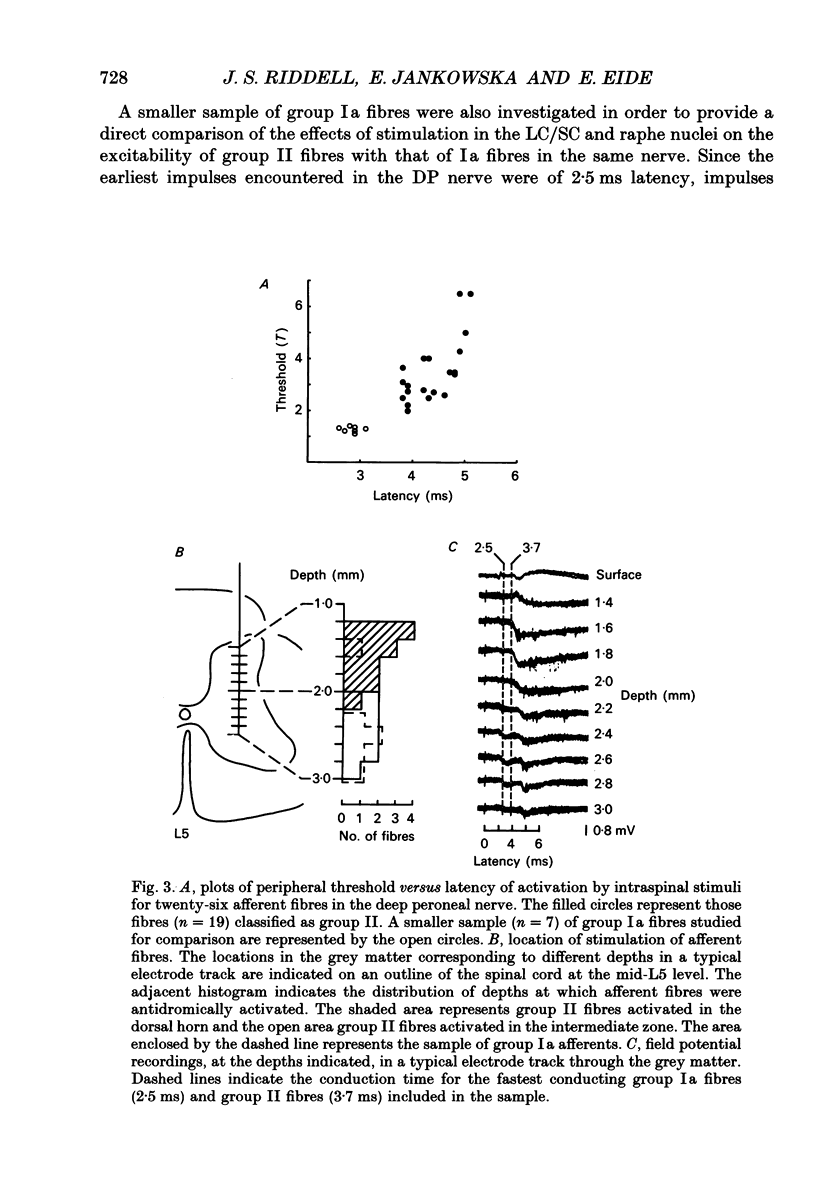

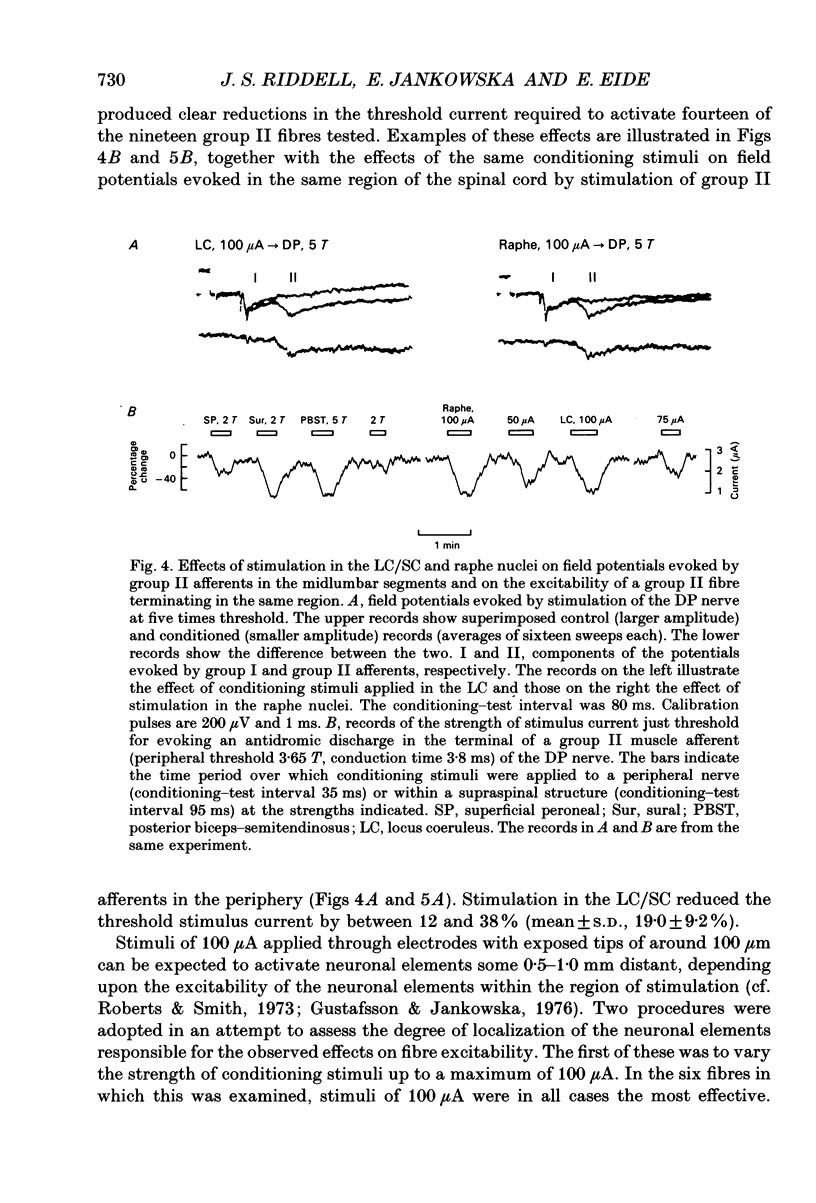









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apkarian A. V., Hodge C. J., Jr, Stevens R. T., Franck J. I. Lumbar dorsal root potentials elicited by stimulation of nucleus locus coeruleus. Exp Neurol. 1984 Jul;85(1):202–208. doi: 10.1016/0014-4886(84)90173-0. [DOI] [PubMed] [Google Scholar]
- Bras H., Cavallari P., Jankowska E., Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol. 1989 Dec 1;290(1):1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- Bras H., Jankowska E., Noga B., Skoog B. Comparison of Effects of Various Types of NA and 5-HT Agonists on Transmission from Group II Muscle Afferents in the Cat. Eur J Neurosci. 1990;2(12):1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- CARPENTER D., LUNDBERG A., NORRSELL U. PRIMARY AFFERENT DEPOLARIZATION EVOKED FROM THE SENSORIMOTOR CORTEX. Acta Physiol Scand. 1963 Sep-Oct;59:126–142. doi: 10.1111/j.1748-1716.1963.tb02729.x. [DOI] [PubMed] [Google Scholar]
- Carpenter D., Engberg I., Lundberg A. Primary afferent depolarization evoked from the brain stem and the cerebellum. Arch Ital Biol. 1966 Mar;104(1):73–85. [PubMed] [Google Scholar]
- Cavallari P., Edgley S. A., Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987 Aug;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. A., Jr, Cangiano A., Pompeiano O. Vestibular control of transmission in primary afferents to the lumbar spinal cord. Arch Ital Biol. 1969 Aug;107(3):296–320. [PubMed] [Google Scholar]
- Curtis D. R. A method for continuously monitoring the electrical threshold of single intraspinal nerve fibers. Electroencephalogr Clin Neurophysiol. 1979 Oct;47(4):503–506. doi: 10.1016/0013-4694(79)90167-6. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Leah J. D., Peet M. J. Effects of noradrenaline and 5-hydroxytryptamine on spinal Ia afferent terminations. Brain Res. 1983 Jan 10;258(2):328–332. doi: 10.1016/0006-8993(83)91160-5. [DOI] [PubMed] [Google Scholar]
- Dostrovsky J. O., Sessle B. J., Hu J. W. Presynaptic excitability changes produced in brain stem endings of tooth pulp afferents by raphe and other central and peripheral influences. Brain Res. 1981 Aug 10;218(1-2):141–160. doi: 10.1016/0006-8993(81)91297-x. [DOI] [PubMed] [Google Scholar]
- Doyle C. A., Maxwell D. J. Catecholaminergic innervation of the spinal dorsal horn: a correlated light and electron microscopic analysis of tyrosine hydroxylase-immunoreactive fibres in the cat. Neuroscience. 1991;45(1):161–176. doi: 10.1016/0306-4522(91)90112-2. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern R., Harrison P. J., Riddell J. S. The dorsal column projection of muscle afferent fibres from the cat hindlimb. J Physiol. 1988 Jul;401:97–113. doi: 10.1113/jphysiol.1988.sp017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S. J., Barnes C. D. Presynaptic facilitatory action of locus coeruleus stimulation upon hindlimb sensory impulse transmission in decerebrate cats. Arch Ital Biol. 1987 Jul;125(3):187–200. [PubMed] [Google Scholar]
- Fung S. J., Reddy V. K., Bowker R. M., Barnes C. D. Differential labeling of the vestibular complex following unilateral injections of horseradish peroxidase into the cat and rat locus coeruleus. Brain Res. 1987 Jan 20;401(2):347–352. doi: 10.1016/0006-8993(87)91419-3. [DOI] [PubMed] [Google Scholar]
- Gharagozloo A., Holohean A. M., Hackman J. C., Davidoff R. A. Serotonin and GABA-induced depolarizations of frog primary afferent fibers. Brain Res. 1990 Nov 5;532(1-2):19–24. doi: 10.1016/0006-8993(90)91736-z. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976 Jun;258(1):33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihira S., Senba E., Yoshida S., Tohyama M., Yoshiya I. Fine structure of noradrenergic terminals and their synapses in the rat spinal dorsal horn: an immunohistochemical study. Brain Res. 1990 Aug 27;526(1):73–80. doi: 10.1016/0006-8993(90)90251-6. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Jami L., Jankowska E. Further evidence for synaptic actions of muscle spindle secondaries in the middle lumbar segments of the cat spinal cord. J Physiol. 1988 Aug;402:671–686. doi: 10.1113/jphysiol.1988.sp017228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Primary afferent depolarization of central terminals of group II muscle afferents in the cat spinal cord. J Physiol. 1989 Apr;411:71–83. doi: 10.1113/jphysiol.1989.sp017561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. 3. Effects on primary afferent terminals. Exp Brain Res. 1972;15(1):39–53. doi: 10.1007/BF00234957. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. I. Effects on alpha-motoneurones innervating hindlimb muscles in cats. Exp Brain Res. 1969;7(4):344–364. doi: 10.1007/BF00237320. [DOI] [PubMed] [Google Scholar]
- Hu J. W., Sessle B. J. Properties of functionally identified nociceptive and nonnociceptive facial primary afferents and presynaptic excitability changes induced in their brain stem endings by raphe and orofacial stimuli in cats. Exp Neurol. 1988 Sep;101(3):385–399. doi: 10.1016/0014-4886(88)90050-7. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Riddell J. S., Skoog B., Noga B. R. Gating of transmission to motoneurones by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol. 1993 Feb;461:705–722. doi: 10.1113/jphysiol.1993.sp019537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez I., Rudomin P., Solodkin M. PAD patterns of physiologically identified afferent fibres from the medial gastrocnemius muscle. Exp Brain Res. 1988;71(3):643–657. doi: 10.1007/BF00248758. [DOI] [PubMed] [Google Scholar]
- Lovick T. A. Primary afferent depolarization of tooth pulp afferents by stimulation in nucleus raphe magnus and the adjacent reticular formation in the cat: effects of bicuculline. Neurosci Lett. 1981 Sep 1;25(2):173–178. doi: 10.1016/0304-3940(81)90327-x. [DOI] [PubMed] [Google Scholar]
- Lucier G. E., Sessle B. J. Presynaptic excitability changes induced in the solitary tract endings of laryngeal primary afferents by stimulation of nucleus raphe magnus and locus coeruleus. Neurosci Lett. 1981 Nov 4;26(3):221–226. doi: 10.1016/0304-3940(81)90136-1. [DOI] [PubMed] [Google Scholar]
- MacLennan C. R. The behaviour of receptors of extramuscular and muscular origin with afferent fibres contributing to the group I and the group II of the cat tibialis anterior muscle nerve. J Physiol. 1972 Apr;222(1):90P–91P. [PubMed] [Google Scholar]
- Madrid J., Alvarado J., Dutton H., Rudomín P. A method for the dynamic continuous estimation of excitability changes of single fiber terminals in the central nervous system. Neurosci Lett. 1979 Mar;11(3):253–258. doi: 10.1016/0304-3940(79)90003-x. [DOI] [PubMed] [Google Scholar]
- Martin R. F., Haber L. H., Willis W. D. Primary afferent depolarization of identified cutaneous fibers following stimulation in medial brain stem. J Neurophysiol. 1979 May;42(3):779–790. doi: 10.1152/jn.1979.42.3.779. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Léránth C., Verhofstad A. A. Synaptic arrangements formed by serotonin-immunoreactive axons in the substantia gelatinosa of the rat spinal cord. Q J Exp Physiol. 1985 Jul;70(3):377–388. doi: 10.1113/expphysiol.1985.sp002923. [DOI] [PubMed] [Google Scholar]
- Mokha S. S., Iggo A. Mechanisms mediating the brain stem control of somatosensory transmission in the dorsal horn of the cat's spinal cord: an intracellular analysis. Exp Brain Res. 1987;69(1):93–106. doi: 10.1007/BF00247032. [DOI] [PubMed] [Google Scholar]
- Mokha S. S., McMillan J. A., Iggo A. Pathways mediating descending control of spinal nociceptive transmission from the nuclei locus coeruleus (LC) and raphe magnus (NRM) in the cat. Exp Brain Res. 1986;61(3):597–606. doi: 10.1007/BF00237586. [DOI] [PubMed] [Google Scholar]
- Noga B. R., Bras H., Jankowska E. Transmission from group II muscle afferents is depressed by stimulation of locus coeruleus/subcoeruleus, Kölliker-Fuse and raphe nuclei in the cat. Exp Brain Res. 1992;88(3):502–516. doi: 10.1007/BF00228180. [DOI] [PubMed] [Google Scholar]
- Proudfit H. K., Anderson E. G. New long latency bulbospinal evoked potentials blocked by serotonin antagonists. Brain Res. 1974 Jan 18;65(3):542–546. doi: 10.1016/0006-8993(74)90246-7. [DOI] [PubMed] [Google Scholar]
- Proudfit H. K., Larson A. A., Anderson E. G. The role of GABA and serotonin in the mediation of raphe-evoked spinal cord dorsal root potentials. Brain Res. 1980 Aug 11;195(1):149–165. doi: 10.1016/0006-8993(80)90873-2. [DOI] [PubMed] [Google Scholar]
- Roberts W. J., Smith D. O. Analysis of threshold currents during microstimulation of fibres in the spinal cord. Acta Physiol Scand. 1973 Nov;89(3):384–394. doi: 10.1111/j.1748-1716.1973.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Ruda M. A., Coffield J., Steinbusch H. W. Immunocytochemical analysis of serotonergic axons in laminae I and II of the lumbar spinal cord of the cat. J Neurosci. 1982 Nov;2(11):1660–1671. doi: 10.1523/JNEUROSCI.02-11-01660.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P., Solodkin M., Jiménez I. PAD and PAH response patterns of group Ia- and Ib-fibers to cutaneous and descending inputs in the cat spinal cord. J Neurophysiol. 1986 Oct;56(4):987–1006. doi: 10.1152/jn.1986.56.4.987. [DOI] [PubMed] [Google Scholar]
- Rudomín P., Jiménez I., Solodkin M., Dueñas S. Sites of action of segmental and descending control of transmission on pathways mediating PAD of Ia- and Ib-afferent fibers in cat spinal cord. J Neurophysiol. 1983 Oct;50(4):743–769. doi: 10.1152/jn.1983.50.4.743. [DOI] [PubMed] [Google Scholar]
- Sasa M., Takaori S. Influence of the locus coeruleus on transmission in the spinal trigeminal nucleus neurons. Brain Res. 1973 May 30;55(1):203–208. doi: 10.1016/0006-8993(73)90502-7. [DOI] [PubMed] [Google Scholar]
- Skoog B., Noga B. R. Do noradrenergic descending tract fibres contribute to the depression of transmission from group II muscle afferents following brainstem stimulation in the cat? Neurosci Lett. 1991 Dec 16;134(1):5–8. doi: 10.1016/0304-3940(91)90495-f. [DOI] [PubMed] [Google Scholar]
- Todd A. J., Millar J. Receptive fields and responses to ionophoretically applied noradrenaline and 5-hydroxytryptamine of units recorded in laminae I-III of cat dorsal horn. Brain Res. 1983 Dec 12;288(1-2):159–167. doi: 10.1016/0006-8993(83)90090-2. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Yassir N., Fleetwood-Walker S. M., Mitchell R. Heterogeneous effects of serotonin in the dorsal horn of rat: the involvement of 5-HT1 receptor subtypes. Brain Res. 1988 Jul 19;456(1):147–158. doi: 10.1016/0006-8993(88)90356-3. [DOI] [PubMed] [Google Scholar]


