Abstract
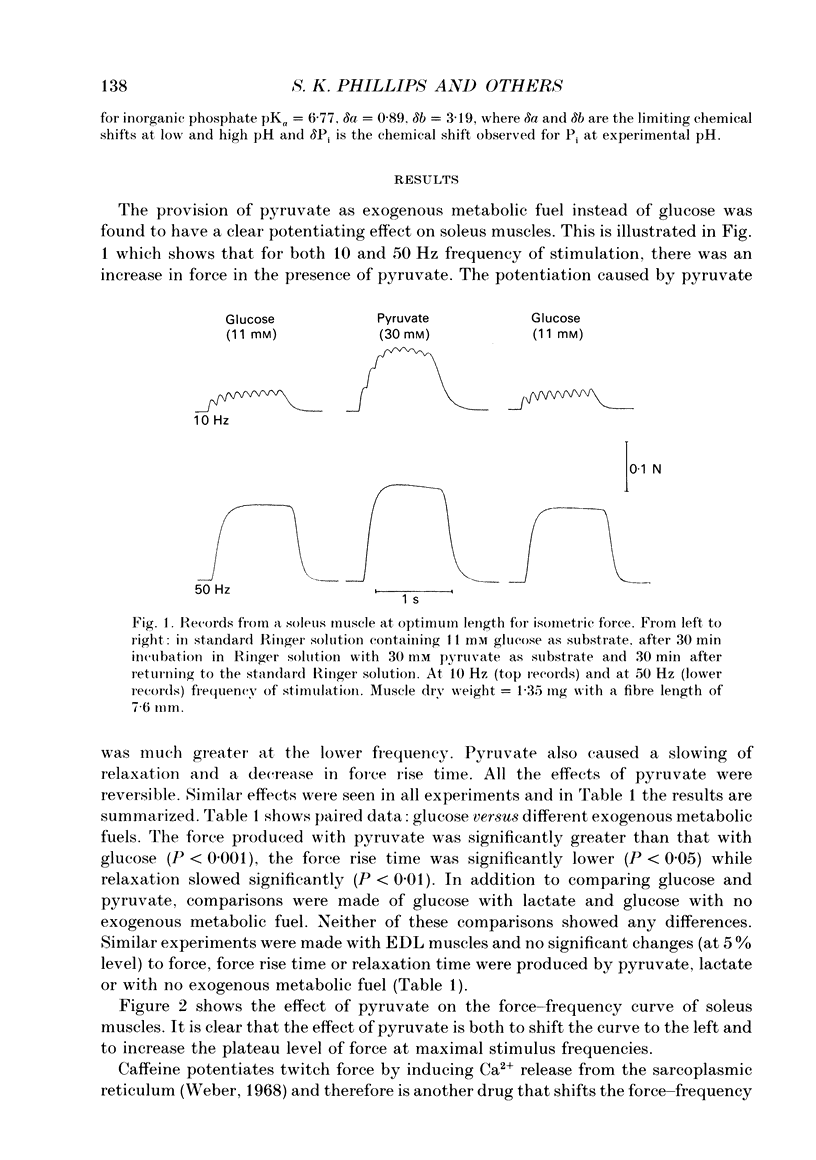
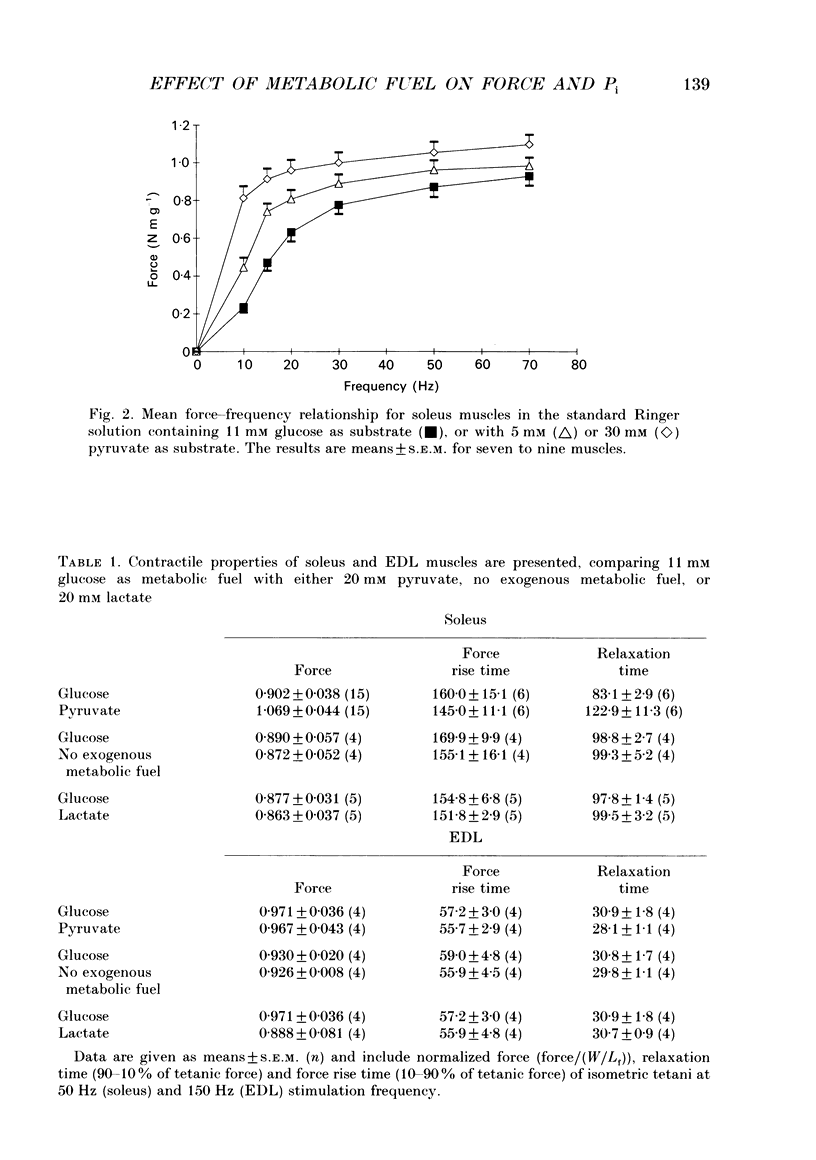
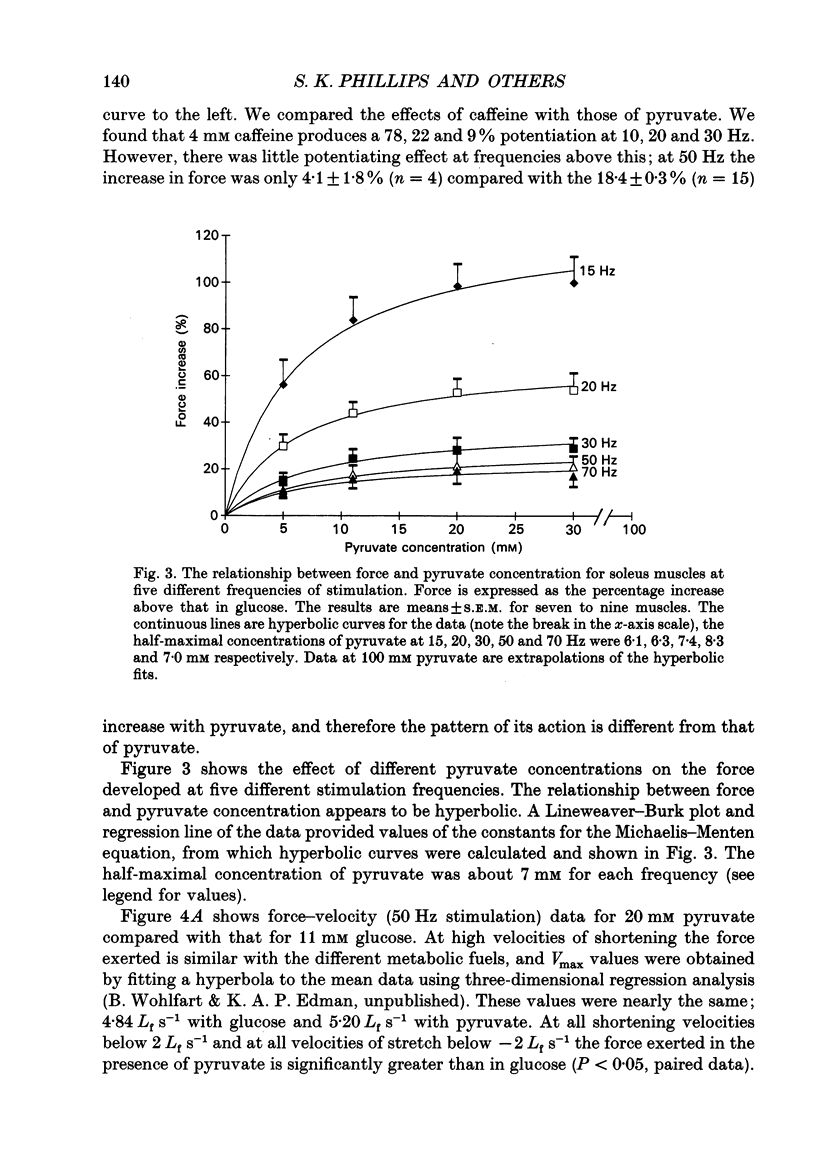
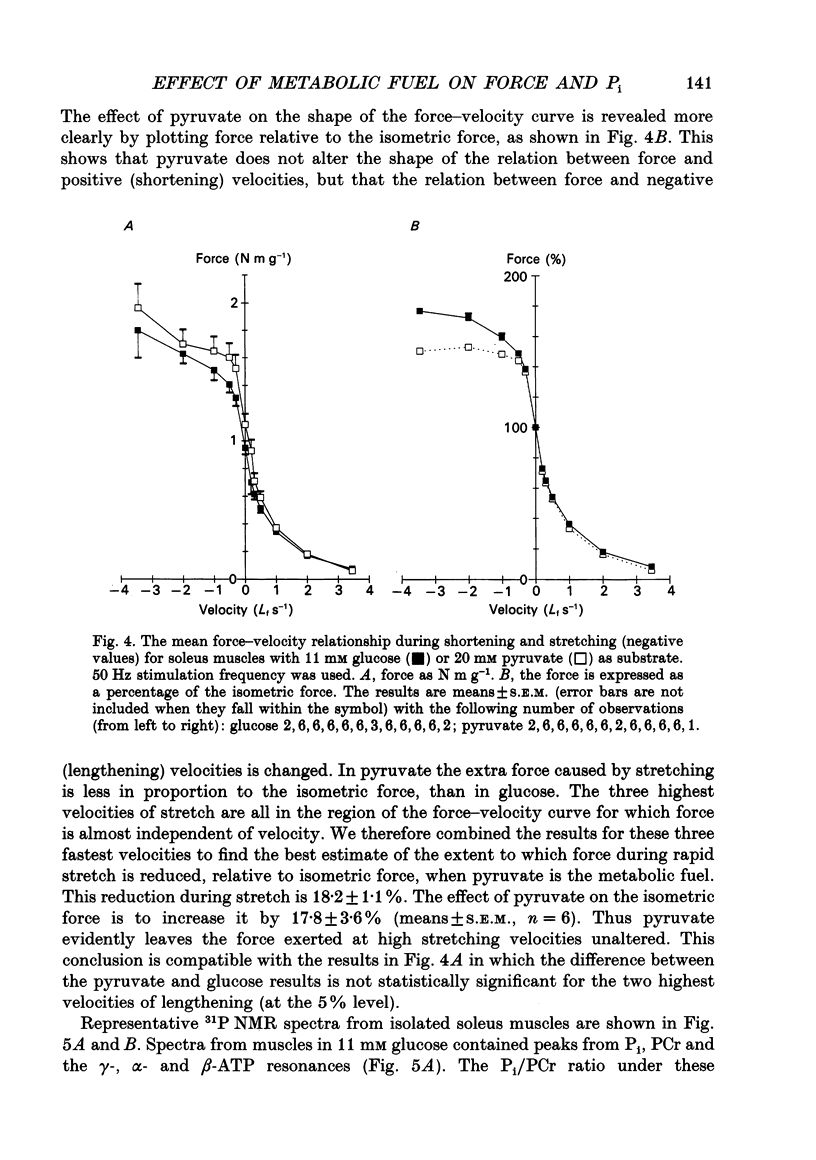
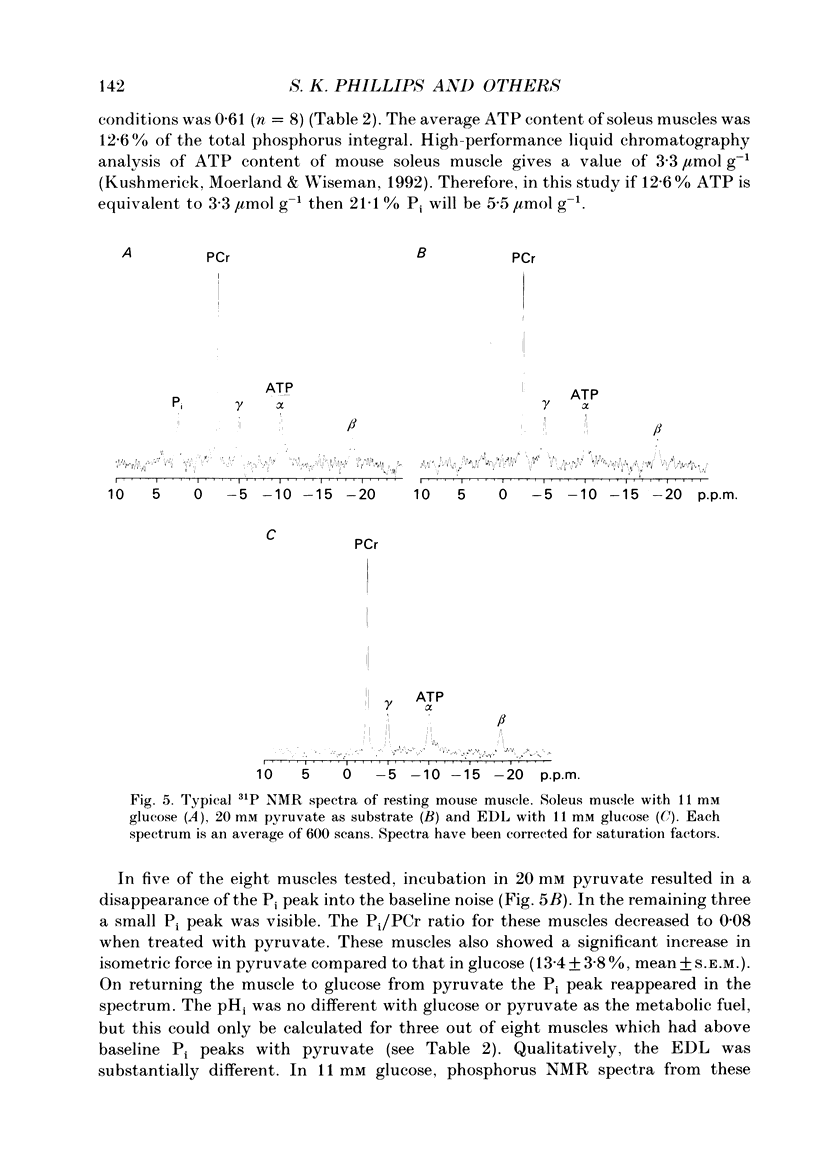
1. The effect of different metabolic fuels (glucose, pyruvate and lactate) and no exogenous metabolic fuel on force production was studied in isolated mouse soleus and extensor digitorum longus (EDL) muscles. Force was measured, at 25 degrees C, during isometric tetanic contractions and during contractions with isovelocity stretching and shortening. In parallel experiments, measurements were made of the resting phosphorus metabolite levels using 31P NMR. 2. In soleus muscles, the isometric tetanic force was potentiated with pyruvate (20 mM) as metabolic fuel, compared with glucose (11 mM), by 17.8 +/- 3.6% (mean +/- S.E.M., n = 6). The force was the same with no exogenous metabolic fuel, with glucose, or with lactate as metabolic fuel. The force exerted during shortening was also potentiated by pyruvate and by the same proportion as isometric force. However, during rapid stretching there was no force enhancement with pyruvate. The changes in the force seen with pyruvate are qualitatively similar to those produced when inorganic phosphate (Pi) is lowered in skinned rabbit psoas muscle fibres. 3. We tested whether the Pi content decreased in the presence of pyruvate by measuring resting Pi using 31P NMR spectroscopy. We found that, in soleus muscles, resting Pi was present with glucose and absent with pyruvate as metabolic fuel, and the effect was reversible. 4. EDL muscles produced the same isometric force whether the metabolic fuel was glucose, pyruvate, lactate or if no exogenous metabolic fuel was supplied. EDL muscles already had Pi levels below detectability at rest in glucose. There were no changes in the 31P NMR spectrum with pyruvate as metabolic fuel. 5. It appears therefore that the force potentiation in soleus muscles with pyruvate is due to a lowering of Pi. EDL muscles, which have a very low resting Pi in glucose, therefore have very little potential for force enhancement by this mechanism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Cox R. N., Kawai M., Robinson T. Effect of cross-bridge kinetics on apparent Ca2+ sensitivity. J Gen Physiol. 1982 Jun;79(6):997–1016. doi: 10.1085/jgp.79.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. B., Gibbs C. L. The effect of metabolic substrate on mechanical activity and heart production in papillary muscle. Cardiovasc Res. 1974 Sep;8(5):656–667. doi: 10.1093/cvr/8.5.656. [DOI] [PubMed] [Google Scholar]
- Clark B. J., 3rd, Acker M. A., McCully K., Subramanian H. V., Hammond R. L., Salmons S., Chance B., Stephenson L. W. In vivo 31P-NMR spectroscopy of chronically stimulated canine skeletal muscle. Am J Physiol. 1988 Feb;254(2 Pt 1):C258–C266. doi: 10.1152/ajpcell.1988.254.2.C258. [DOI] [PubMed] [Google Scholar]
- Daut J., Elzinga G. Substrate dependence of energy metabolism in isolated guinea-pig cardiac muscle: a microcalorimetric study. J Physiol. 1989 Jun;413:379–397. doi: 10.1113/jphysiol.1989.sp017659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Moerland T. S., Wiseman R. W. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7521–7525. doi: 10.1073/pnas.89.16.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuoka H., Weisfeldt M. L., Zweier J. L., Jacobus W. E., Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986 Sep;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Kushmerick M. J. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985 Mar;248(3 Pt 1):C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem. 1990 Nov 25;265(33):20234–20240. [PubMed] [Google Scholar]
- Passonneau J. V., Lowry O. H. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul. 1964;2:265–274. doi: 10.1016/s0065-2571(64)80018-2. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. J Muscle Res Cell Motil. 1989 Jun;10(3):181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- Phillips S. K., Bruce S. A., Woledge R. C. In mice, the muscle weakness due to age is absent during stretching. J Physiol. 1991 Jun;437:63–70. doi: 10.1113/jphysiol.1991.sp018583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uğurbil K., Kingsley-Hickman P. B., Sako E. Y., Zimmer S., Mohanakrishnan P., Robitaille P. M., Thoma W. J., Johnson A., Foker J. E., From A. H. 31P NMR studies of the kinetics and regulation of oxidative phosphorylation in the intact myocardium. Ann N Y Acad Sci. 1987;508:265–286. doi: 10.1111/j.1749-6632.1987.tb32910.x. [DOI] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968 Nov;52(5):760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. The relative isometric strength of type I and type II muscle fibres in the human quadriceps. Clin Physiol. 1984 Feb;4(1):23–32. doi: 10.1111/j.1475-097x.1984.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Jacobus W. E. Substrate-induced alterations of high energy phosphate metabolism and contractile function in the perfused heart. J Biol Chem. 1987 Jun 15;262(17):8015–8021. [PubMed] [Google Scholar]


