Abstract
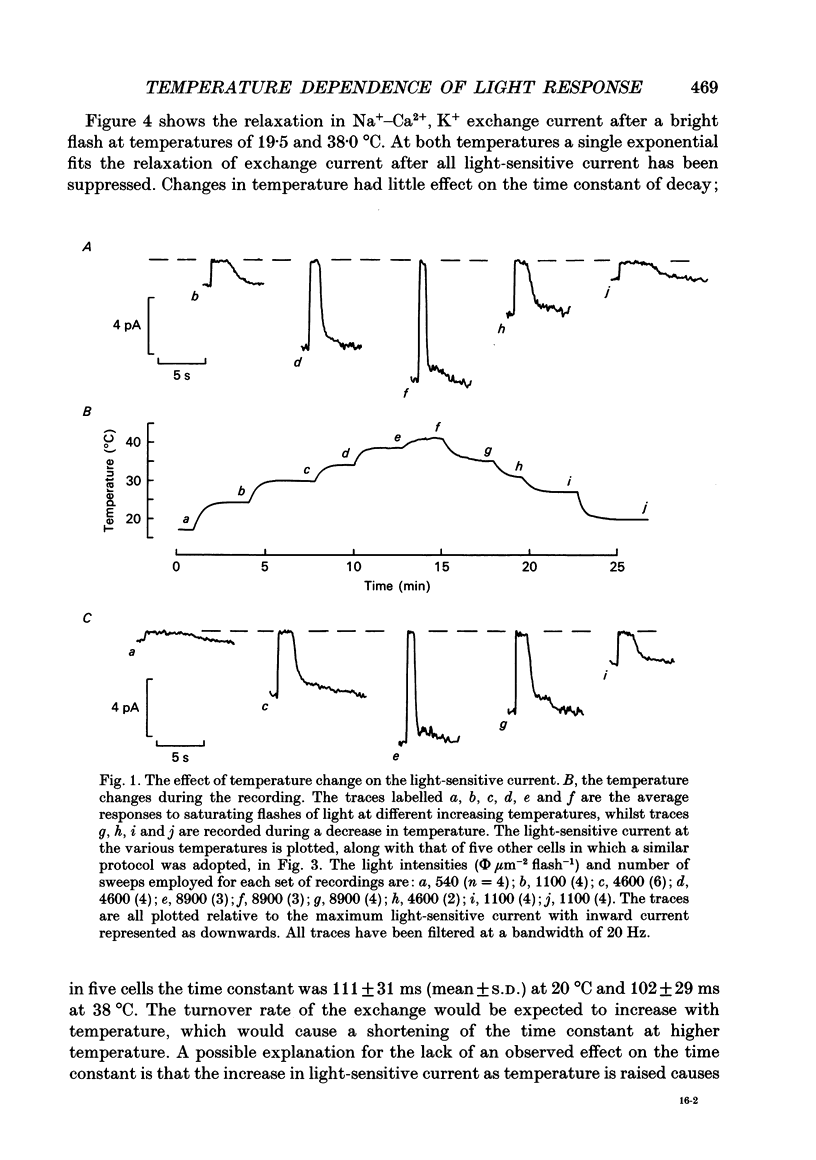
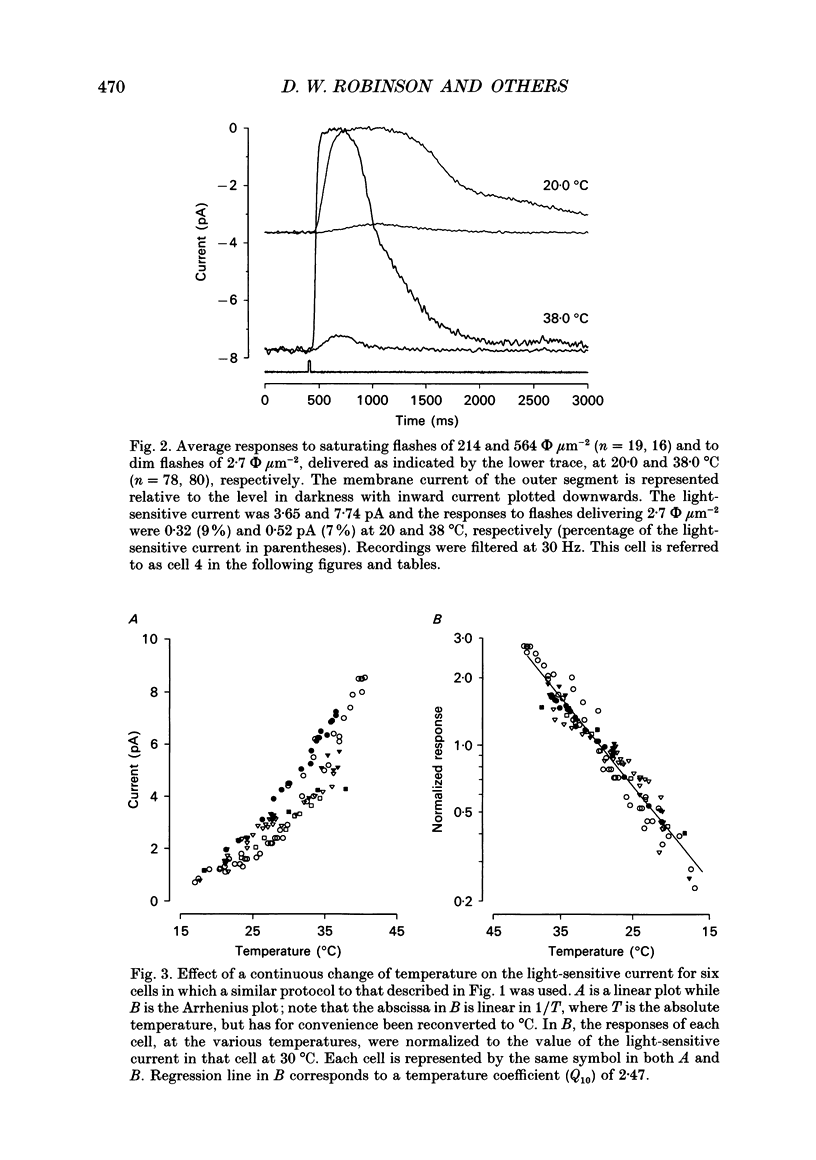
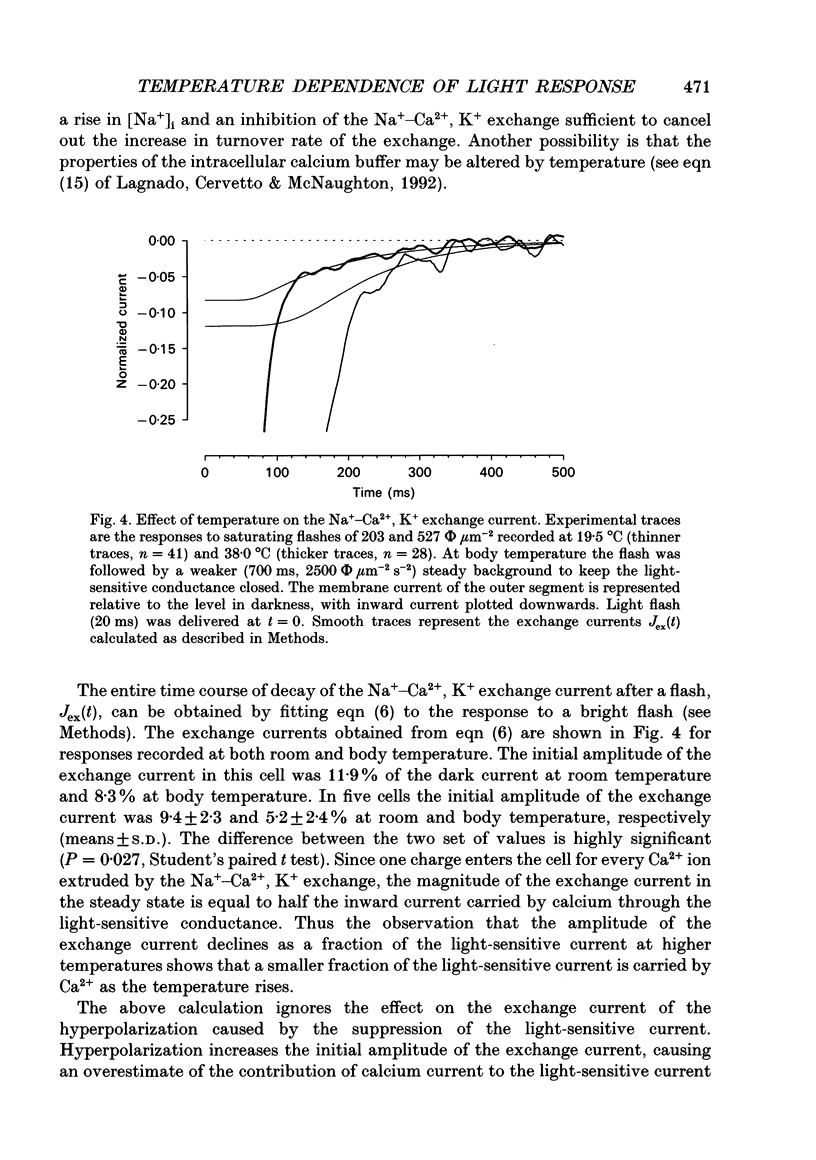
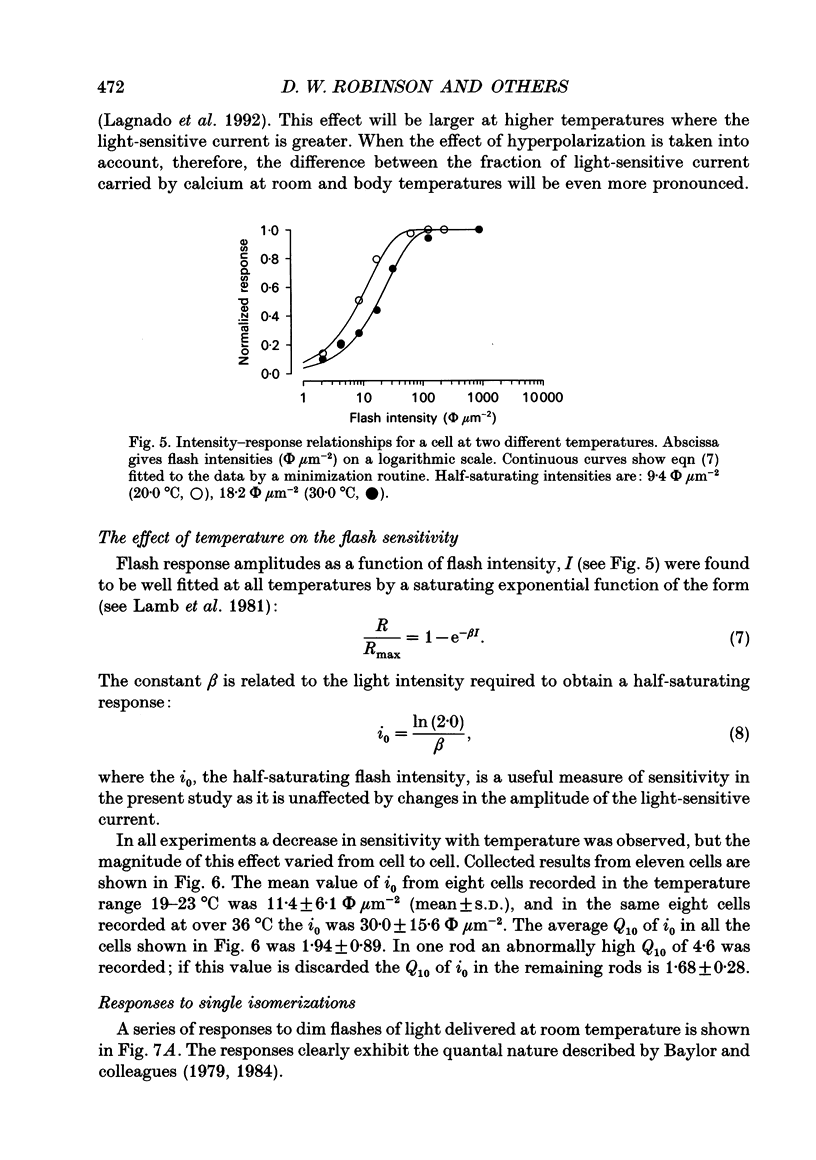
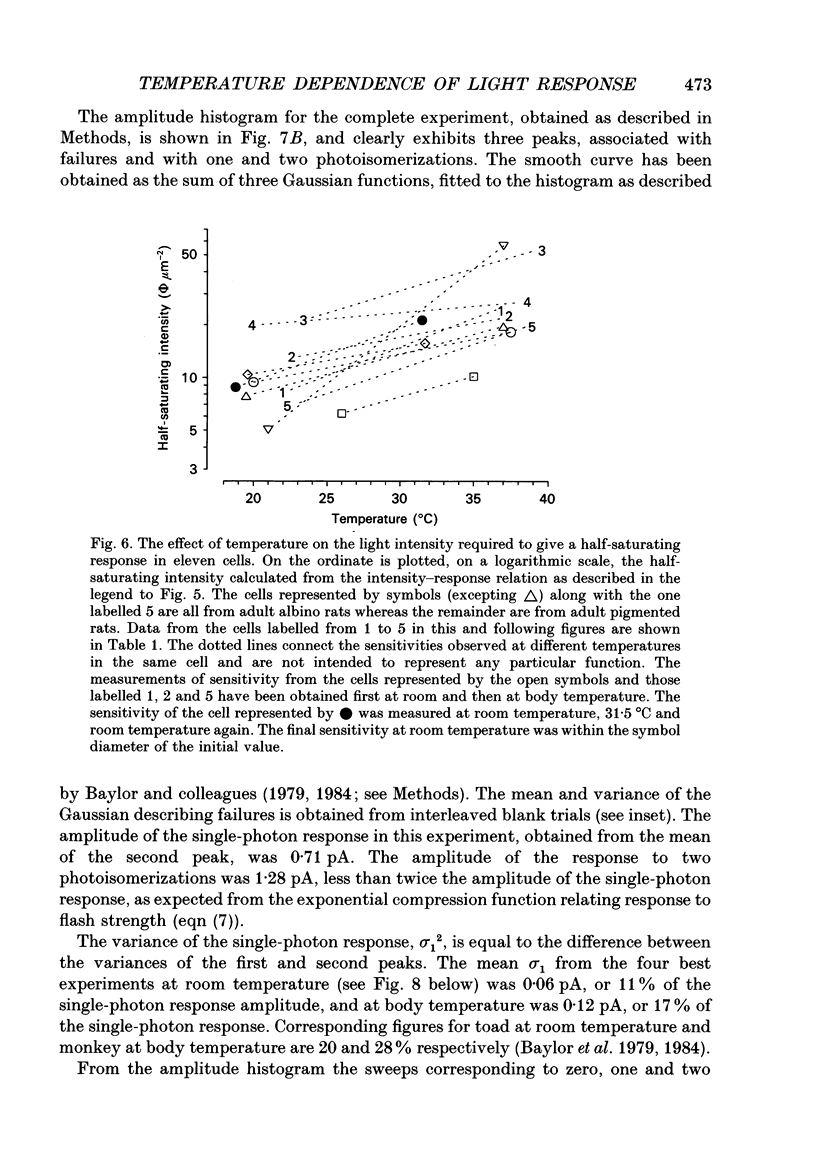
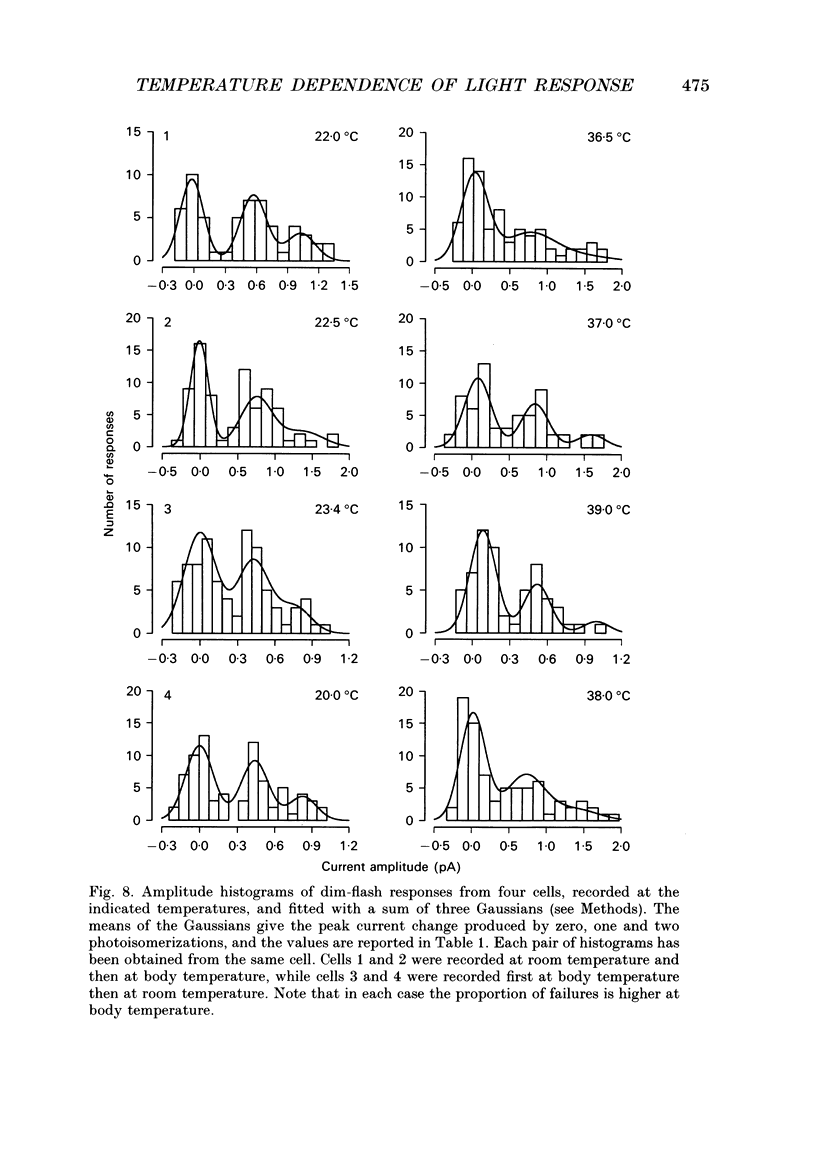
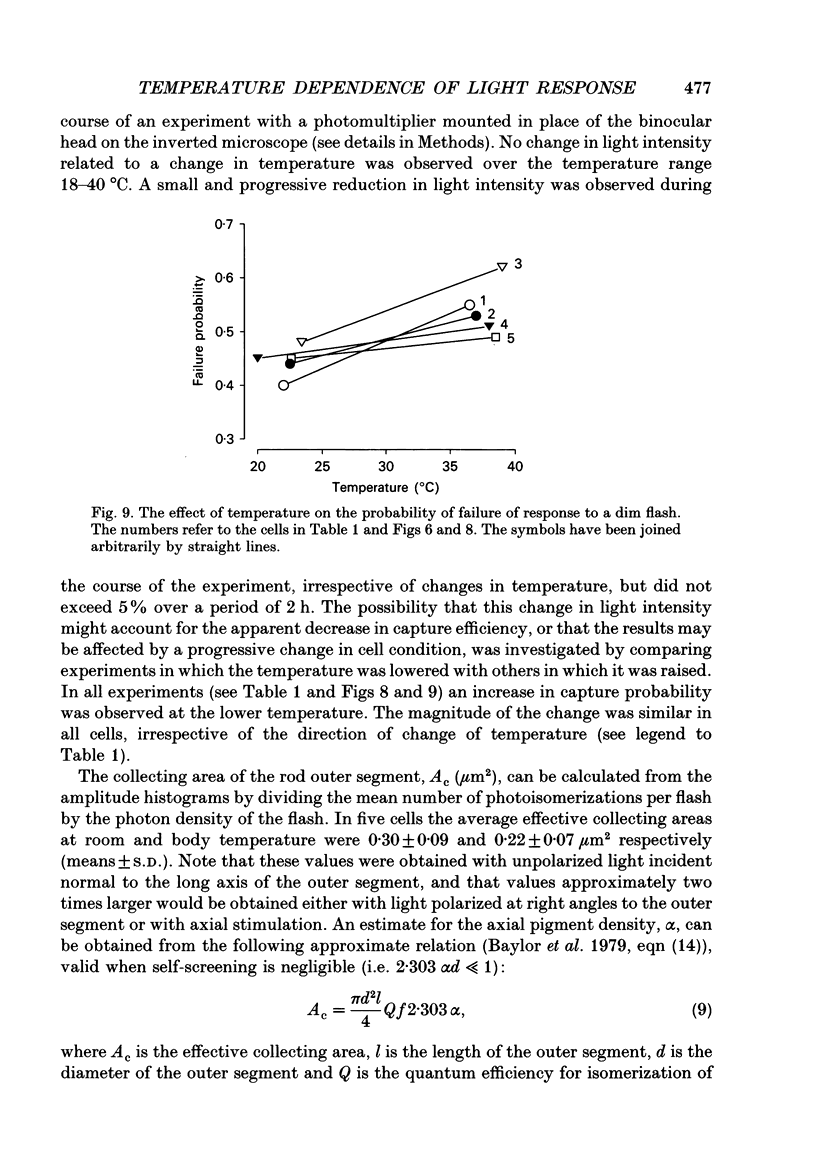
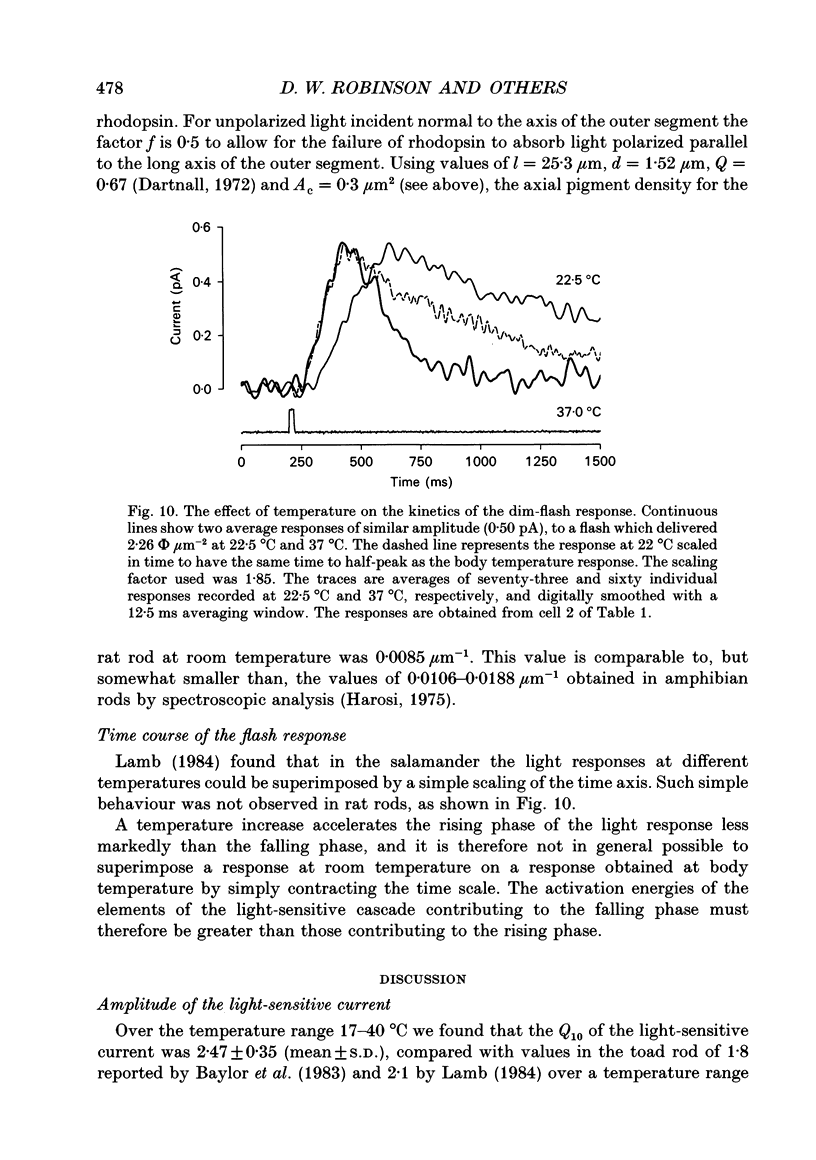
1. The effects of temperature on the light responses of rat rods have been investigated over the range 17-40 degrees C. 2. The amplitude of the light-sensitive current increased with temperature with a mean temperature coefficient (Q10) of 2.47. 3. The amplitude of the Na(+)-Ca2+, K+ exchange current decreased with temperature when expressed as a fraction of the light-sensitive current, showing that the light-sensitive channel becomes less permeable to calcium as the temperature is raised. The time constant of relaxation of the exchange current was little affected by temperature. 4. The flash intensity required to give a half-saturating response increased with temperature with a mean Q10 of 1.68. 5. The responses to single photoisomerizations were determined from amplitude histograms of the responses to dim-flash trains. The amplitude of the response to a single photoisomerization decreased with temperature when expressed as a fraction of the light-sensitive current, but the change was not sufficient to account for the overall decrease in sensitivity. 6. The fraction of dim flashes that produced a photoisomerization decreased with temperature. This decrease in photon capture efficiency together with the decrease in the relative size of the single photon event fully accounts for the observed change in sensitivity. 7. The speed of the falling phase of the dim-flash response was accelerated more by warming than the rising phase, and it was therefore not possible to superimpose light responses at different temperatures by a simple change in time scale.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Temperature effects on the membrane current of retinal rods of the toad. J Physiol. 1983 Apr;337:723–734. doi: 10.1113/jphysiol.1983.sp014651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., McNaughton P. A. Net charge transport during sodium-dependent calcium extrusion in isolated salamander rod outer segments. J Gen Physiol. 1991 Sep;98(3):479–495. doi: 10.1085/jgp.98.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Effects of temperature changes on toad rod photocurrents. J Physiol. 1984 Jan;346:557–578. doi: 10.1113/jphysiol.1984.sp015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto G. M., Robinson D. W., Yan B., McNaughton P. A. Development of the light response in neonatal mammalian rods. Nature. 1991 Jun 20;351(6328):654–657. doi: 10.1038/351654a0. [DOI] [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol. 1991 Jul;98(1):95–130. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]


