Abstract
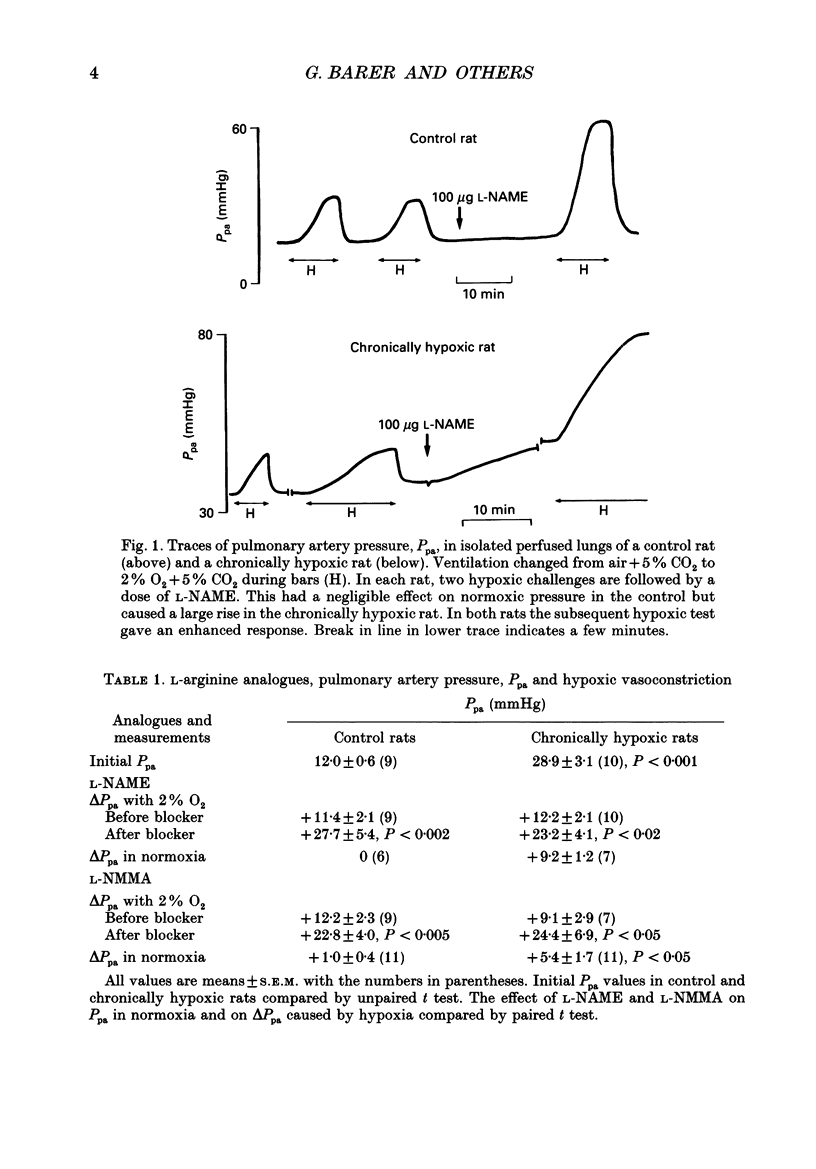
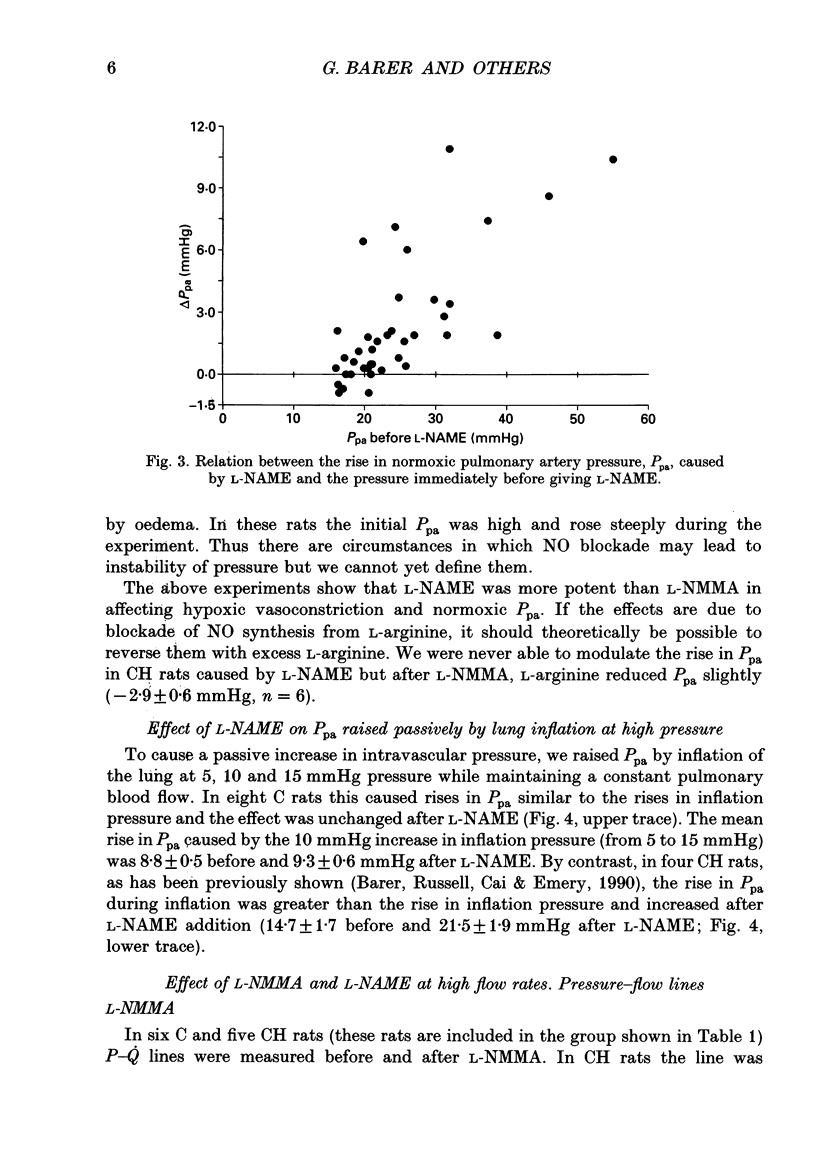
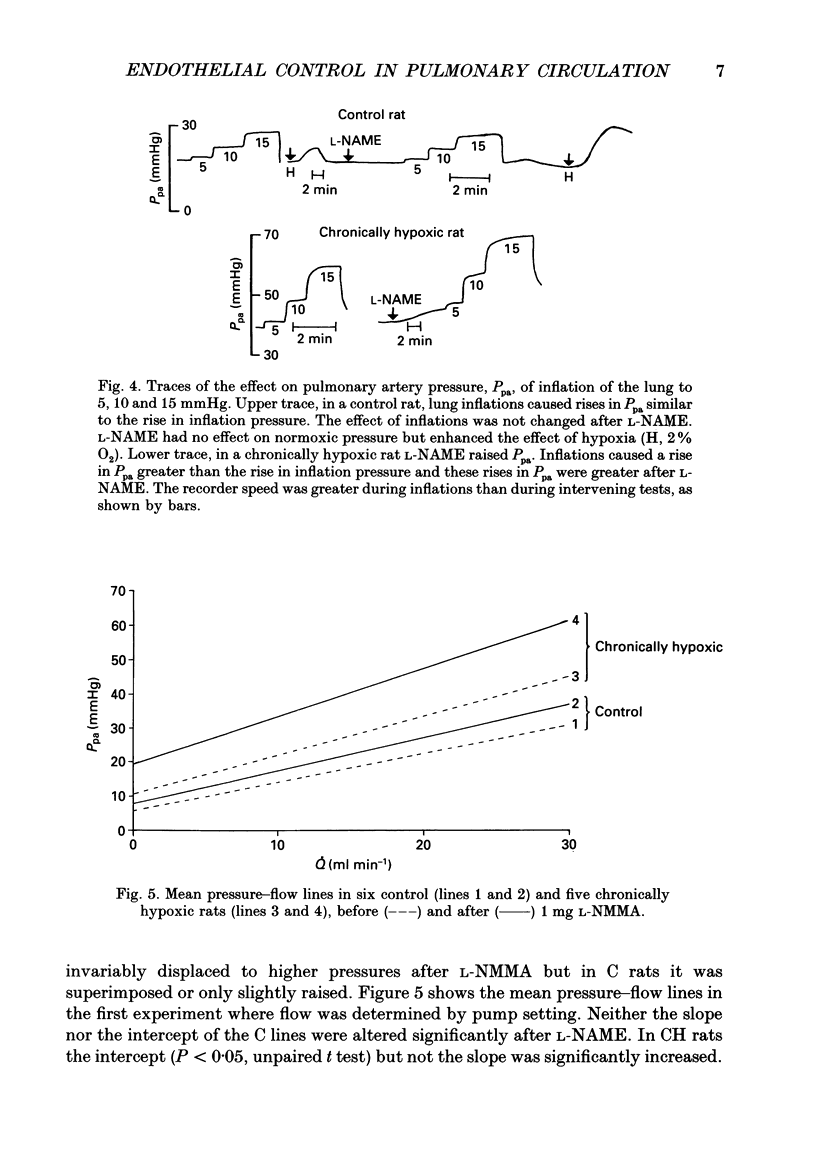
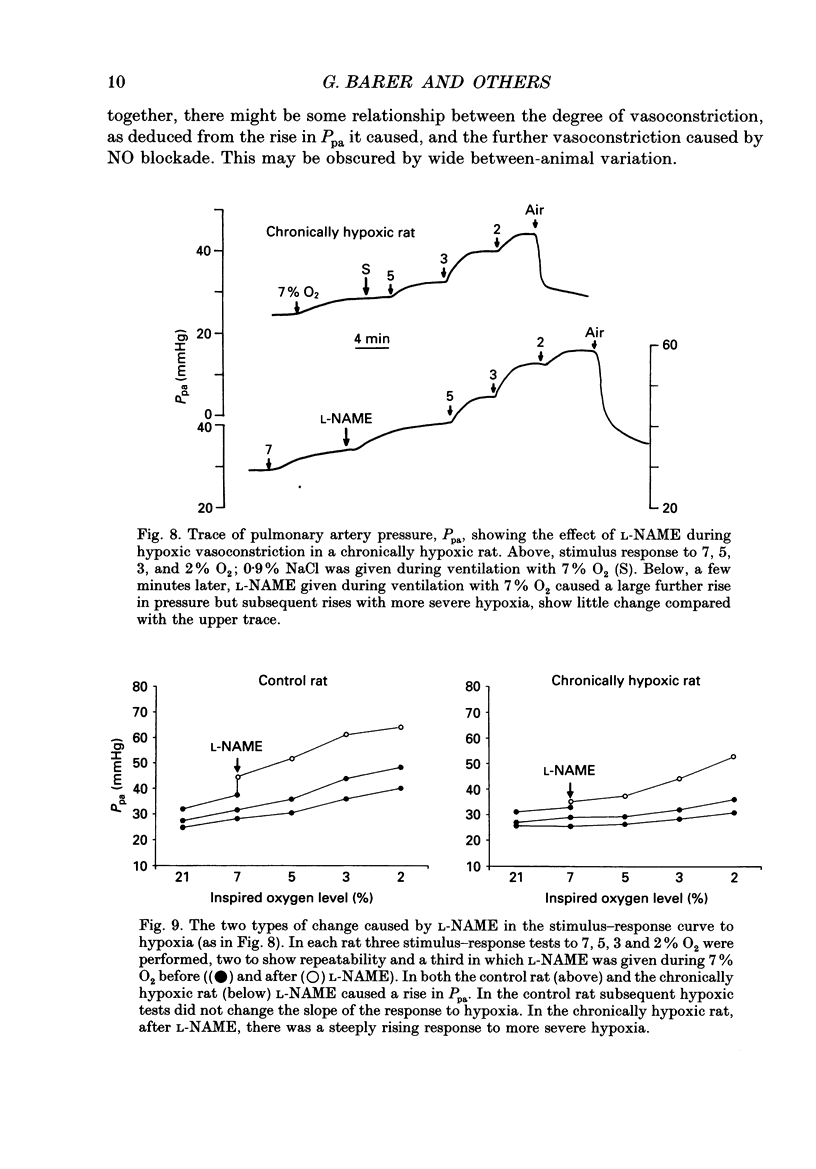
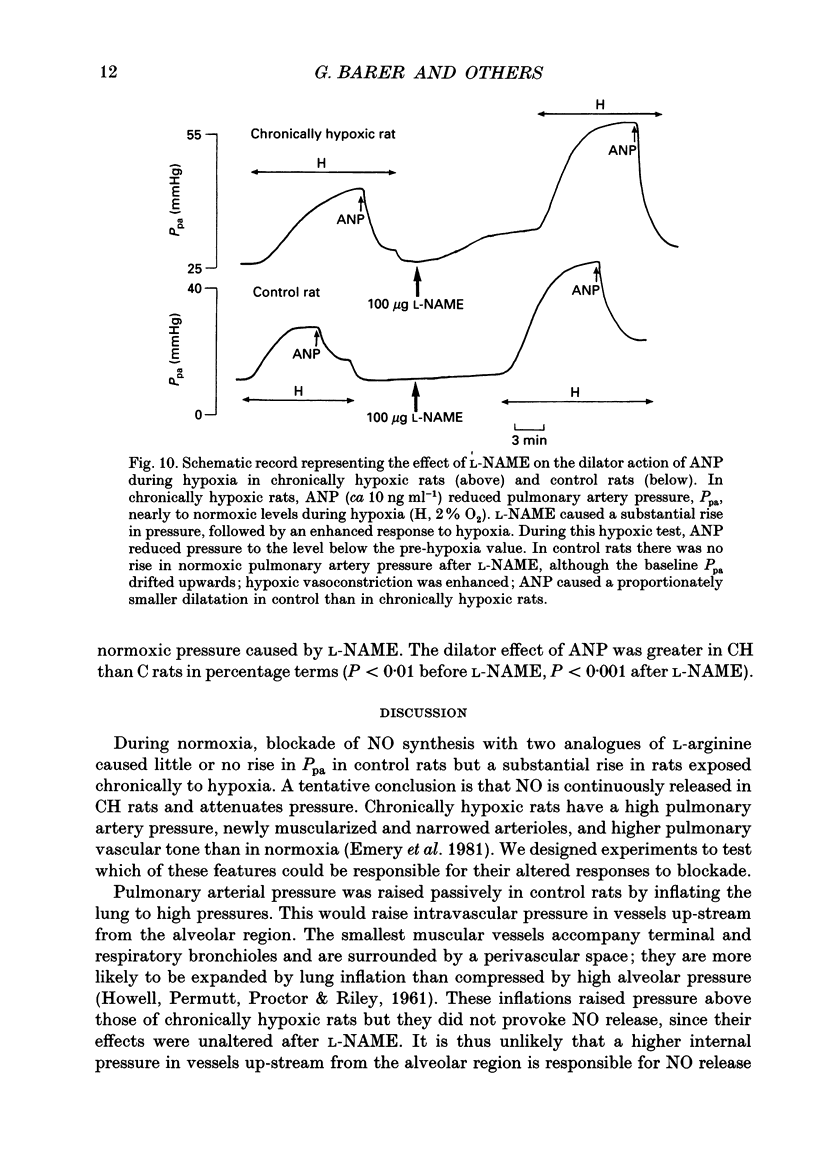
1. The effect of blockade of nitric oxide synthesis in pulmonary endothelium by two L-arginine analogues was tested in isolated blood-perfused lungs of normal rats and rats exposed chronically to 10% O2. 2. In both groups of rats the analogues (N-monomethyl-L-arginine (L-NMMA) and N-nitro-L-arginine methyl ester (L-NAME)) enhanced hypoxic vasoconstriction. In normal rats, with rare exceptions, these analogues had little or no effect on pulmonary artery pressure (Ppa) at constant blood flow during normoxia. However, chronically hypoxic rats have pulmonary hypertension and in these rats the analogues always raised Ppa; the rise in Ppa after L-NMMA but not L-NAME could be partially reversed by L-arginine. L-NAME was more potent than L-NMMA. 3. To see whether the difference between rat groups was due to the high Ppa in chronically hypoxic rats, in control rats we raised Ppa passively by lung inflation to values higher than found in chronically hypoxic rats. L-NAME did not alter the effects of lung inflation on Ppa. 4. Ppa was also raised passively by plotting pressure-flow lines up to high flow rates; the lines were changed minimally by both analogues in control rats but in chronically hypoxic rats the lines were raised to higher pressures and steepened substantially. 5. In control rats, during vasoconstriction caused by hypoxia, endothelin 1 and almitrine, L-NAME caused further rises in pressure. We conclude that a stimulus for nitric oxide release in control rats is the narrowing of vessels caused by vasoconstriction rather than passive increases in intravascular pressure. 6. In chronically hypoxic rats arterioles are narrowed by growth of new muscle and there is some muscle tone even in normoxia. Thus narrowing of the vascular lumen is the stimulus common to both groups of rats which leads to nitric oxide synthesis and attenuation of Ppa by a negative feedback process. Narrowing is associated with a large increase in shear stress due to two factors; the pressure drop along a vessel segment is increased and the surface area of the lining of the affected segment is decreased. 7. Atrial natriuretic peptide caused dose-dependent pulmonary vasodilation in both rat groups but had a greater effect in chronically hypoxic rats. The action persisted and was enhanced after blockade of NO synthesis.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adnot S., Raffestin B., Eddahibi S., Braquet P., Chabrier P. E. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J Clin Invest. 1991 Jan;87(1):155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh Xuan A. T., Higenbottam T. W., Pepke-Zaba J., Clelland C., Wallwork J. Reduced endothelium-dependent relaxation of cystic fibrosis pulmonary arteries. Eur J Pharmacol. 1989 Apr 25;163(2-3):401–403. doi: 10.1016/0014-2999(89)90217-3. [DOI] [PubMed] [Google Scholar]
- Dinh-Xuan A. T., Higenbottam T. W., Clelland C. A., Pepke-Zaba J., Cremona G., Butt A. Y., Large S. R., Wells F. C., Wallwork J. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991 May 30;324(22):1539–1547. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- Emery C. J., Bee D., Barer G. R. Mechanical properties and reactivity of vessels in isolated perfused lungs of chronically hypoxic rats. Clin Sci (Lond) 1981 Nov;61(5):569–580. doi: 10.1042/cs0610569. [DOI] [PubMed] [Google Scholar]
- HOWELL J. B., PERMUTT S., PROCTOR D. F., RILEY R. L. Effect of inflation of the lung on different parts of pulmonary vascular bed. J Appl Physiol. 1961 Jan;16:71–76. doi: 10.1152/jappl.1961.16.1.71. [DOI] [PubMed] [Google Scholar]
- JAMESON A. G. GASEOUS DIFFUSION FROM ALVEOLI INTO PULMONARY ARTERIES. J Appl Physiol. 1964 May;19:448–456. doi: 10.1152/jappl.1964.19.3.448. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Robertson B. E., Warren J. B., Nye P. C. Inhibition of nitric oxide synthesis potentiates hypoxic vasoconstriction in isolated rat lungs. Exp Physiol. 1990 Mar;75(2):255–257. doi: 10.1113/expphysiol.1990.sp003399. [DOI] [PubMed] [Google Scholar]
- Weir E. K. Does normoxic pulmonary vasodilatation rather than hypoxic vasoconstriction account for the pulmonary pressor response to hypoxia? Lancet. 1978 Mar 4;1(8062):476–477. doi: 10.1016/s0140-6736(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Wiklund N. P., Persson M. G., Gustafsson L. E., Moncada S., Hedqvist P. Modulatory role of endogenous nitric oxide in pulmonary circulation in vivo. Eur J Pharmacol. 1990 Aug 21;185(1):123–124. doi: 10.1016/0014-2999(90)90221-q. [DOI] [PubMed] [Google Scholar]
- Wilkinson M., Langhorne C. A., Heath D., Barer G. R., Howard P. A pathophysiological study of 10 cases of hypoxic cor pulmonale. Q J Med. 1988 Jan;66(249):65–85. [PubMed] [Google Scholar]


