Abstract
1. Measurements were made of stable (hb) and labile (ha) maintenance heat rate, slowing of relaxation as a function of tetanus duration, and parvalbumin (PA) content in intact single muscle fibres of types 1 and 2 from Xenopus laevis. The majority of experiments were performed at 20 degrees C. In addition, total and myofibrillar ATPase activity was measured in skinned Xenopus fibres, also of types 1 and 2; these studies were performed at 4 degrees C. 2. In agreement with a previous study hb was significantly higher in type 1 (175 +/- 13 mW (g wet wt)-1; n = 8) than in type 2 fibres (88 +/- 9 mW (g wet wt)-1; n = 7). The value of ha was 236 +/- 22 and 117 +/- 16 mW (g wet wt)-1, respectively (mean +/- S.E.M.). ha decayed with a time constant of 0.27 +/- 0.02 (n = 8) and 0.33 +/- 0.02 s (n = 7). 3. The early relaxation rate of tetanic force, extrapolated to the onset of stimulation (yo + yb; where yo is 'extra' rate of relaxation and yb steady rate) was 85.6 +/- 4.2 s-1 for type 1 fibres (n = 8) and 62.7 +/- 7.3 s-1 for type 2 fibres (n = 7). Relaxation rate at the end of a 1.8 s tetanus (yb) was 29.4 +/- 1.6 and 33.3 +/- 1.5 s-1, respectively; thus, there was more slowing with tetanus duration in type 1 fibres. The time constant for slowing of relaxation with tetanus duration was similar to that for decay of ha. 4. Parvalbumin concentration, [PA], was 0.45 +/- 0.04 mM in type 1 (n = 7) and 0.22 +/- 0.04 mM (n = 7) in type 2 fibres. 5. For individual fibres positive correlations were found between the 'extra' rate of relaxation (yo), labile heat (ha) and [PA]. Significantly more labile heat was liberated than can be accounted for by the enthalpy change of Ca2+ binding to PA. 6. For five fibres (type 1) studied both at 20 and 10 degrees C, the magnitude of slowing of relaxation, expressed as yo/(yo + yb), was 0.58 +/- 0.03 at 20 degrees C and 0.65 +/- 0.03 at 10 degrees C. 7. Both slowing of relaxation and labile heat were depressed in the second of two closely spaced tetani in type 1 fibres. Repriming of both effects followed similar, biphasic time courses and required more than 10 min for completion at 20 degrees C.(ABSTRACT TRUNCATED AT 400 WORDS)
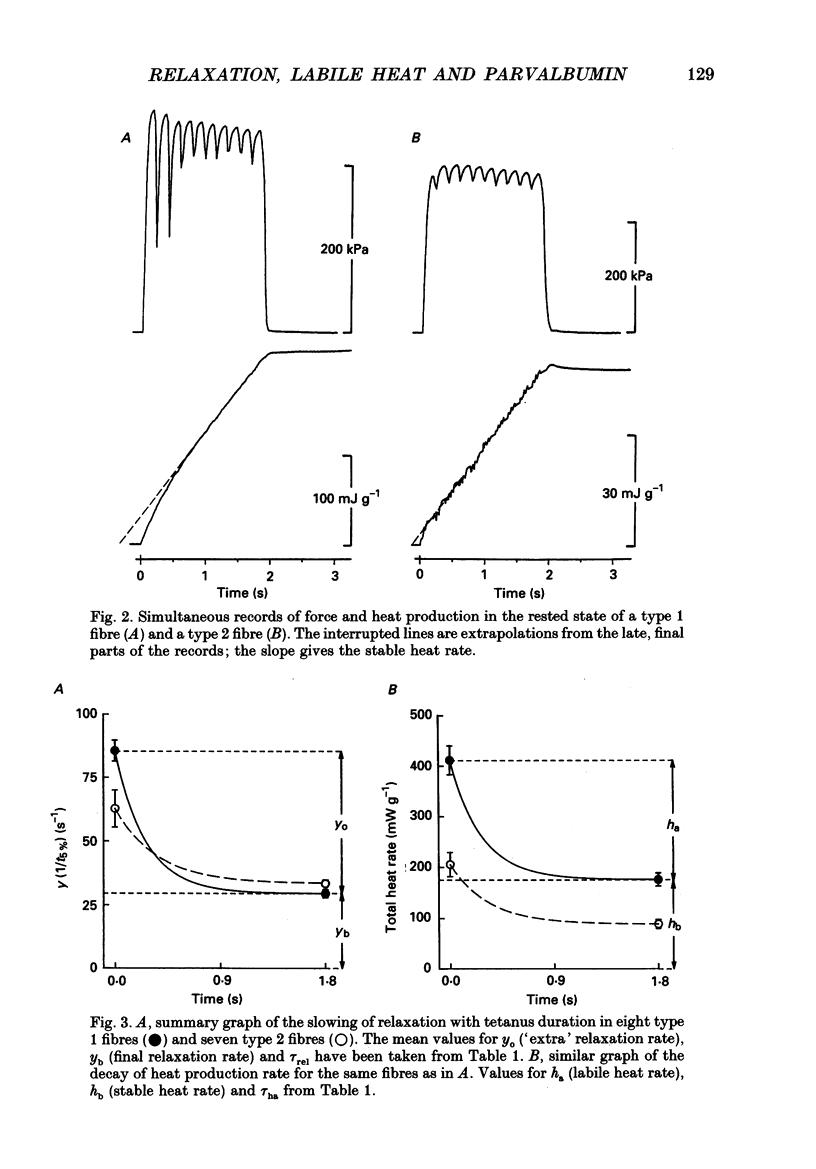
Full text
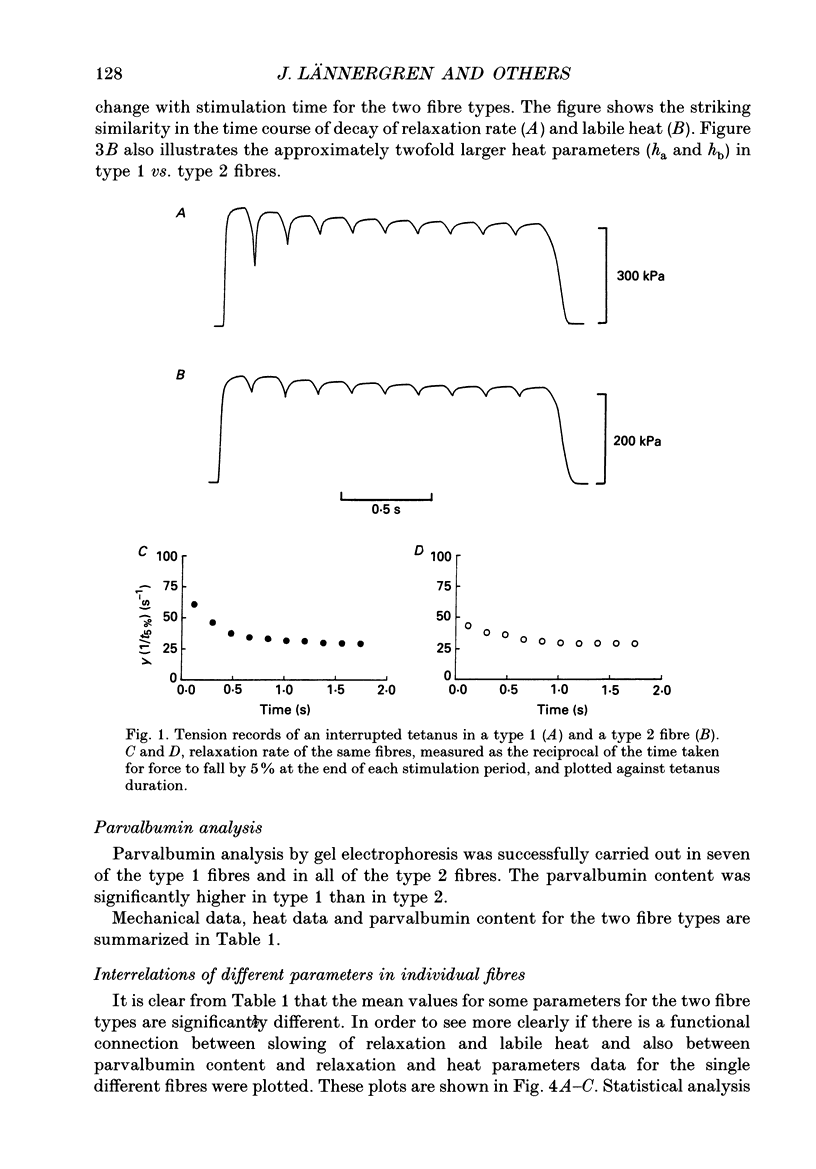
PDF




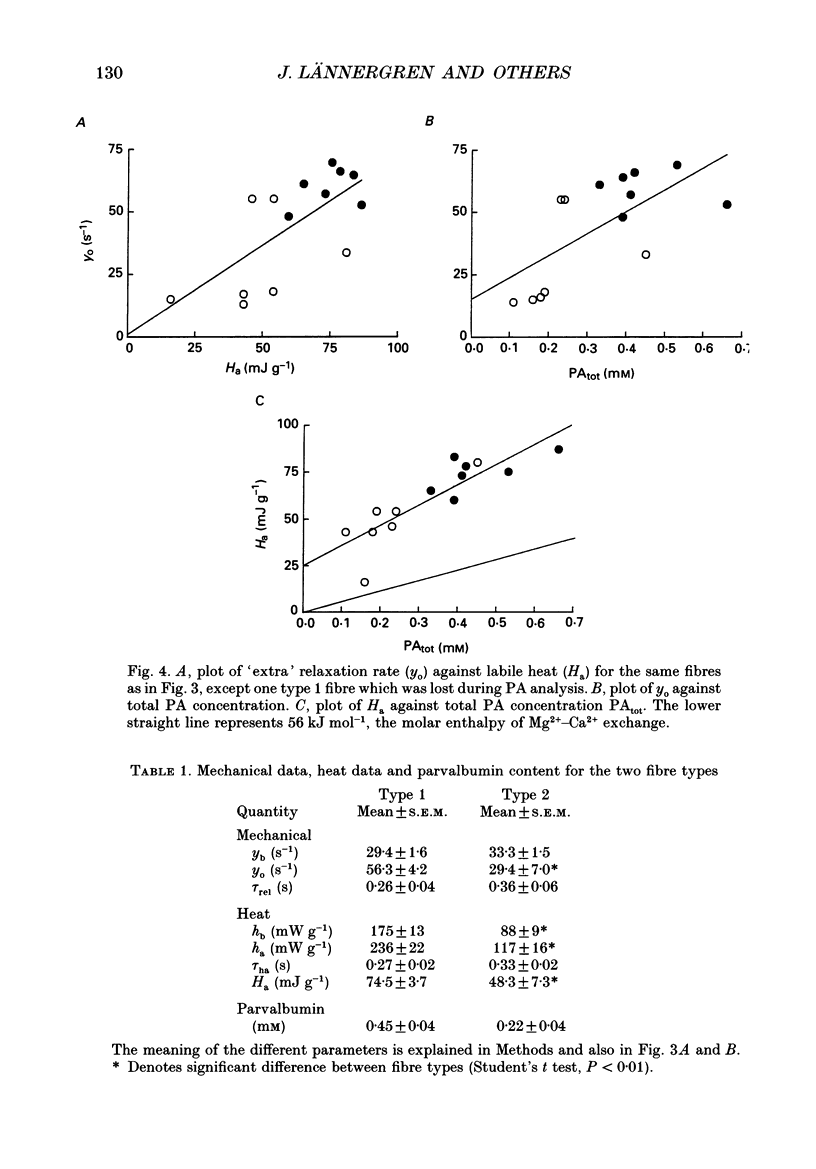

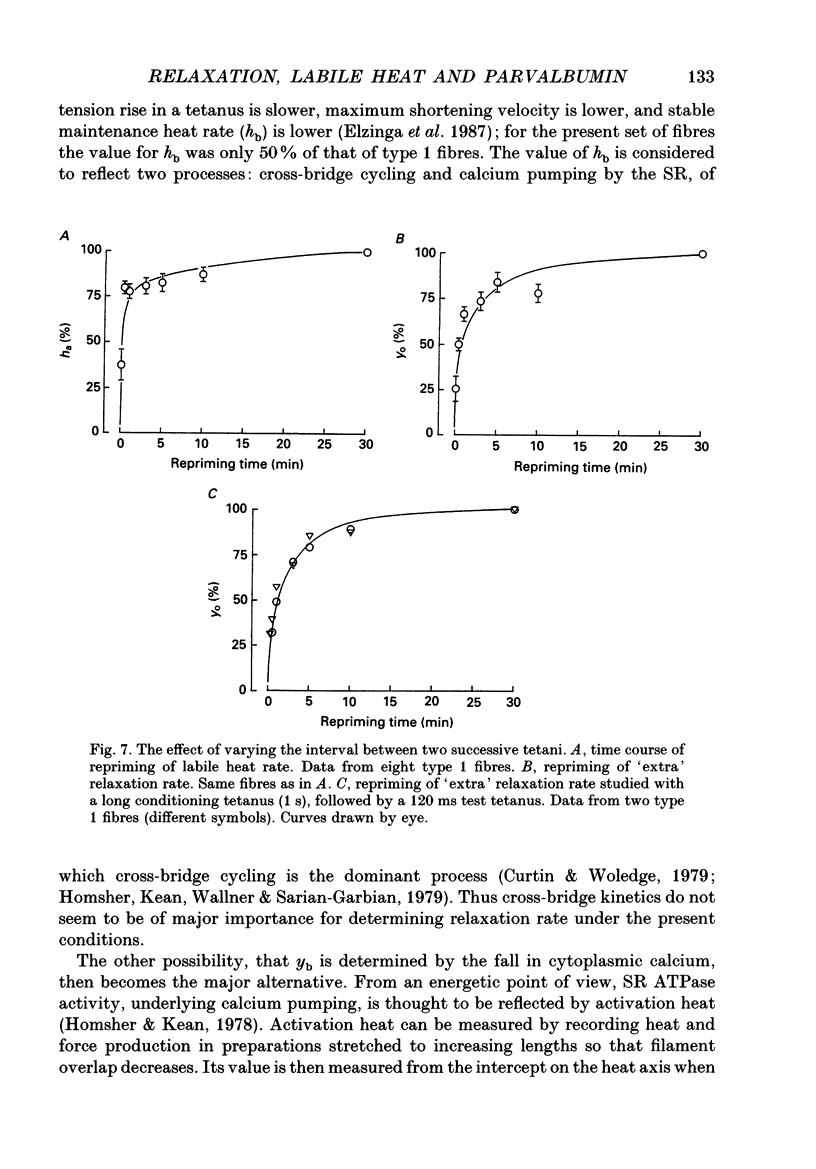
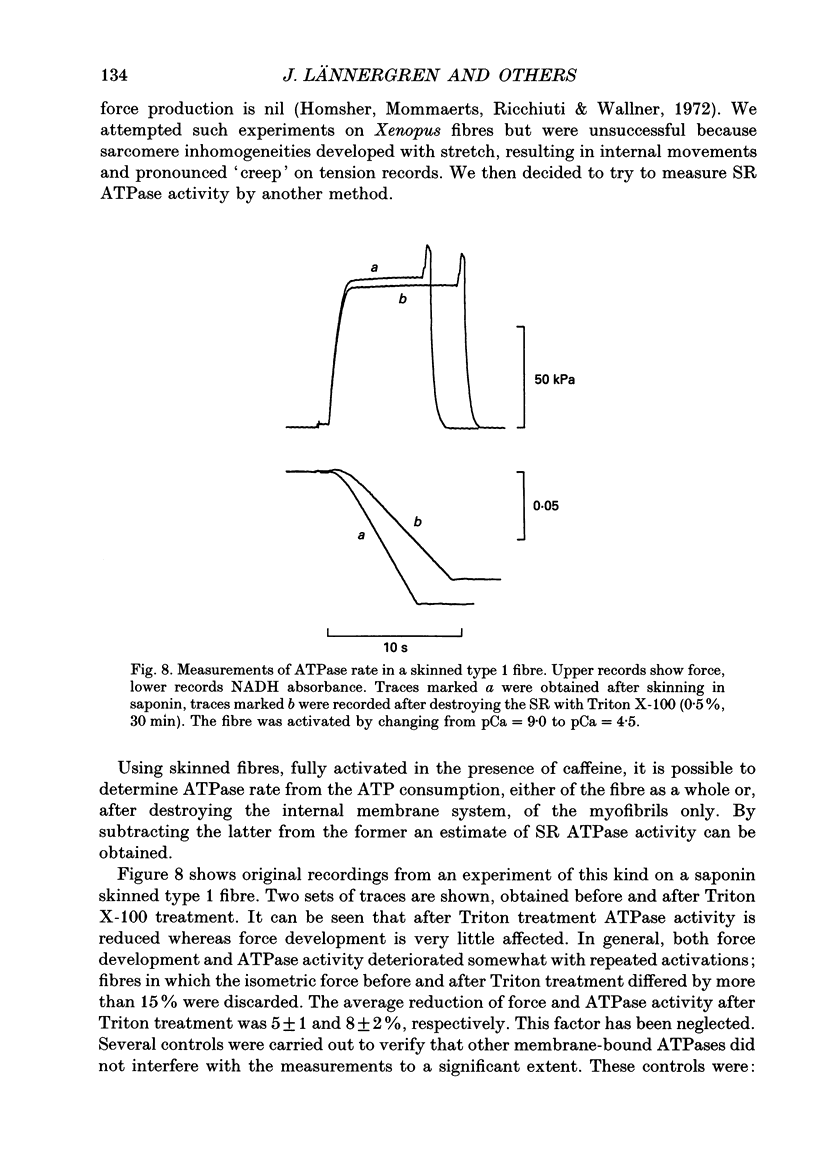
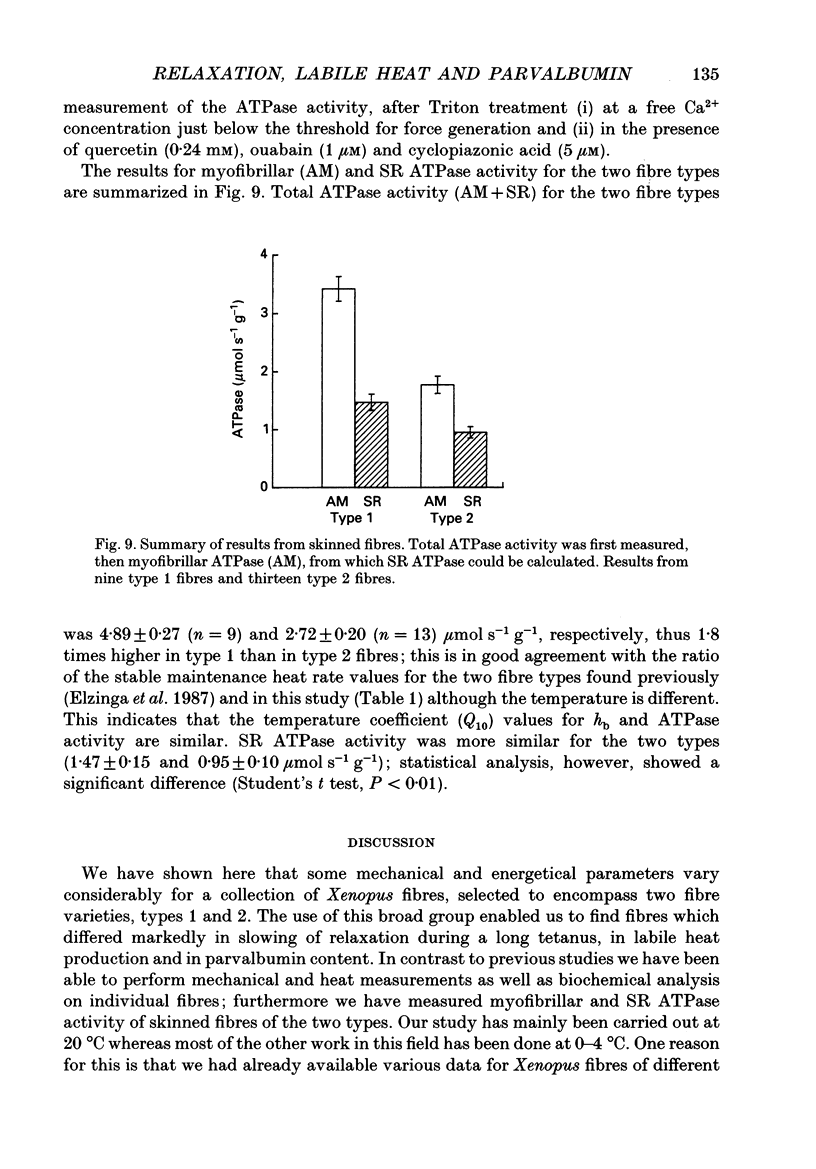





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
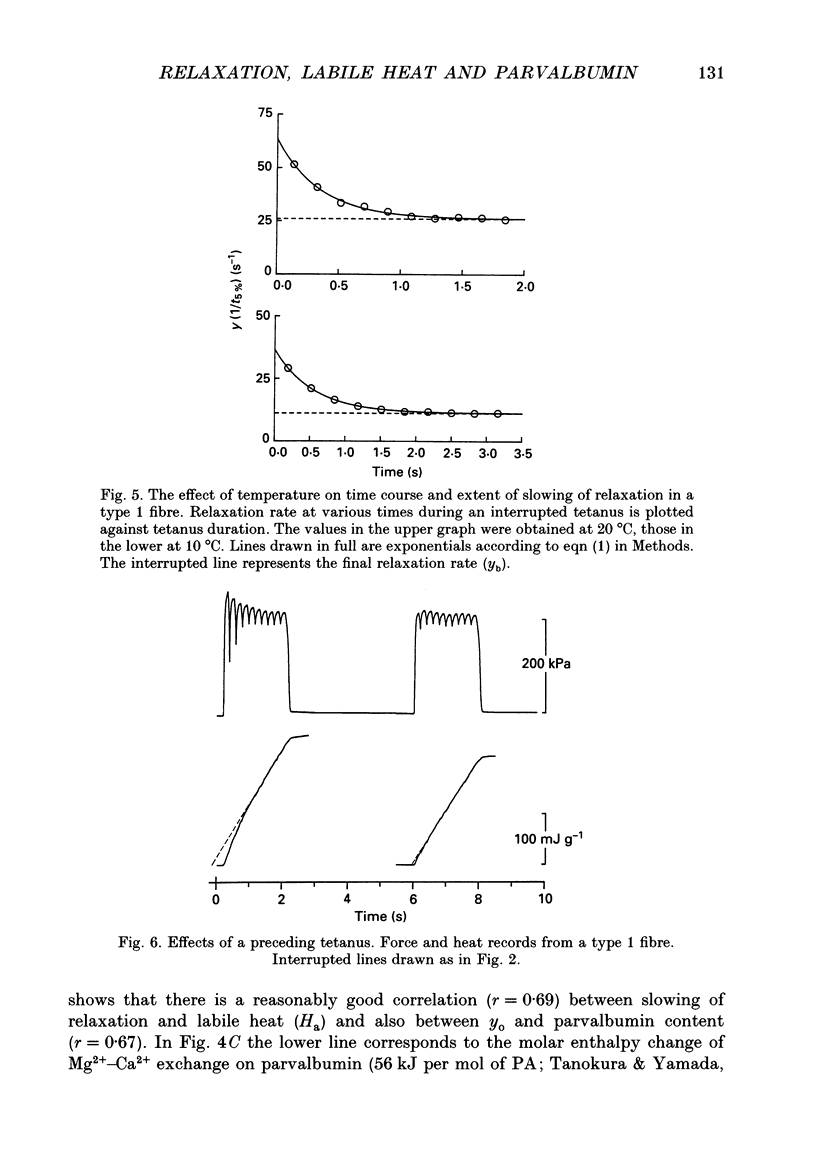
- ABBOTT B. C. The heat production associated with the maintenance of a prolonged contraction and the extra heat produced during large shortening. J Physiol. 1951 Feb;112(3-4):438–445. doi: 10.1113/jphysiol.1951.sp004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin A., Lebacq J. Parvalbumin, labile heat and slowing of relaxation in mouse soleus and extensor digitorum longus muscles. J Physiol. 1992 Jan;445:601–616. doi: 10.1113/jphysiol.1992.sp018942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Rall J. A., Wilson M. G., Woledge R. C. Absolute values of myothermic measurements on single muscle fibres from frog. J Muscle Res Cell Motil. 1986 Aug;7(4):327–332. doi: 10.1007/BF01753653. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Chemical change and energy production during contraction of frog muscle: how are their time courses related? J Physiol. 1979 Mar;288:353–366. [PMC free article] [PubMed] [Google Scholar]
- Elzinga G., Lännergren J., Stienen G. J. Stable maintenance heat rate and contractile properties of different single muscle fibres from Xenopus laevis at 20 degrees C. J Physiol. 1987 Dec;393:399–412. doi: 10.1113/jphysiol.1987.sp016829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glyn H., Sleep J. Dependence of adenosine triphosphatase activity of rabbit psoas muscle fibres and myofibrils on substrate concentration. J Physiol. 1985 Aug;365:259–276. doi: 10.1113/jphysiol.1985.sp015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Kean C. J. Skeletal muscle energetics and metabolism. Annu Rev Physiol. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- Homsher E., Lacktis J., Yamada T., Zohman G. Repriming and reversal of the isometric unexplained enthalpy in frog skeletal muscle. J Physiol. 1987 Dec;393:157–170. doi: 10.1113/jphysiol.1987.sp016817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Mommaerts W. F., Ricchiuti N. V., Wallner A. Activation heat, activation metabolism and tension-related heat in frog semitendinosus muscles. J Physiol. 1972 Feb;220(3):601–625. doi: 10.1113/jphysiol.1972.sp009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Mommaerts W. F., Ricchiuti N. V., Wallner A. Activation heat, activation metabolism and tension-related heat in frog semitendinosus muscles. J Physiol. 1972 Feb;220(3):601–625. doi: 10.1113/jphysiol.1972.sp009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol. 1991 Sep;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano T. Effect of carbon dioxide on heat production of frog skeletal muscles. J Physiol. 1988 Mar;397:643–655. doi: 10.1113/jphysiol.1988.sp017023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. G., Kovacs L., Simon B. J., Schneider M. F. Decline of myoplasmic Ca2+, recovery of calcium release and sarcoplasmic Ca2+ pump properties in frog skeletal muscle. J Physiol. 1991 Sep;441:639–671. doi: 10.1113/jphysiol.1991.sp018771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N., Ogawa Y. Discrimination of Ca(2+)-ATPase activity of the sarcoplasmic reticulum from actomyosin-type ATPase activity of myofibrils in skinned mammalian skeletal muscle fibres: distinct effects of cyclopiazonic acid on the two ATPase activities. J Muscle Res Cell Motil. 1991 Aug;12(4):355–365. doi: 10.1007/BF01738590. [DOI] [PubMed] [Google Scholar]
- Mulieri L. A., Luhr G., Trefry J., Alpert N. R. Metal-film thermopiles for use with rabbit right ventricular papillary muscles. Am J Physiol. 1977 Nov;233(5):C146–C156. doi: 10.1152/ajpcell.1977.233.5.C146. [DOI] [PubMed] [Google Scholar]
- Peckham M., Woledge R. C. Labile heat and changes in rate of relaxation of frog muscles. J Physiol. 1986 May;374:123–135. doi: 10.1113/jphysiol.1986.sp016070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall J. A., Woledge R. C. Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol. 1990 Aug;259(2 Pt 2):R197–R203. doi: 10.1152/ajpregu.1990.259.2.R197. [DOI] [PubMed] [Google Scholar]
- Simonides W. S., van Hardeveld C. Identification and quantification in single muscle fibers of four isoforms of parvalbumin in the iliofibularis muscle of Xenopus laevis. Biochim Biophys Acta. 1989 Oct 5;998(2):137–144. doi: 10.1016/0167-4838(89)90265-3. [DOI] [PubMed] [Google Scholar]
- Stienen G. J., Roosemalen M. C., Wilson M. G., Elzinga G. Depression of force by phosphate in skinned skeletal muscle fibers of the frog. Am J Physiol. 1990 Aug;259(2 Pt 1):C349–C357. doi: 10.1152/ajpcell.1990.259.2.C349. [DOI] [PubMed] [Google Scholar]
- Tanokura M., Yamada K. Heat capacity and entropy changes of the two major isotypes of bullfrog (Rana catesbeiana) parvalbumins induced by calcium binding. Biochemistry. 1987 Dec 1;26(24):7668–7674. doi: 10.1021/bi00398a020. [DOI] [PubMed] [Google Scholar]


