Abstract
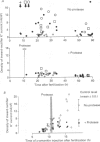
1. Early expression of ion channels following neural induction was examined in isolated, cleavage-arrested blastomeres from the ascidian embryo using a two-electrode voltage clamp. Currents were recorded from the isolated, cleavage-arrested blastomere, a4-2, after treatment with serine protease, subtilisin, which induces neural differentiation as consistently as cell contact. 2. The inward-rectifier K+ current increased at the late gastrula stage shortly after the sensitive period for neural induction both in the induced (protease-treated) and uninduced cells. Ca2+ channels, characteristic of epidermal-type differentiation, and delayed-rectifier K+ channels and differentiated-type Na+ channels, characteristic of neural-type differentiation appeared much later than the inward-rectifier K+ channels, at a time corresponding to the tail bud stage of the intact embryo. 3. When cells were treated with subtilisin during the critical period for neural induction, the increase in the inward-rectifier K+ current from the late gastrula stage to the neurula stage was about three times smaller (3.67 +/- 1.74 nA, mean +/- S.D., n = 14) than in untreated cells (11.25 +/- 3.10 nA, n = 26). The same changes in the inward-rectifier K+ channel were also observed in a4 2 blastomeres which were induced by cell contact with an A4-1 blastomere. However, when cells were treated with subtilisin after the critical period for neural induction, the amplitude of the inward-rectifier K+ current was the same as in untreated cells. Thus the expressed level of the inward-rectifier K+ channel was linked to the determination of neural or epidermal cell types. 4. There was no significant difference in the input capacitance of induced and uninduced cells, indicating that the difference in the amplitude of the inward-rectifier K+ currents derived from a difference in the channel density rather than a difference in cell surface area. 5. The expression of the inward-rectifier K+ channel at the late gastrula stage was sensitive to alpha-amanitin, a highly specific transcription inhibitor. In both induced and uninduced cells, injection of alpha-amanitin at the 32-cell stage reduced the current density of the inward-rectifier K+ channel to about 2 nA/nF, corresponding to 13% of that recorded from uninjected cells. By contrast, the expression of the fast-inactivating-type Na+ current, which transiently increased along with the inward-rectifier K+ channel, was resistant to alpha-amanitin injection. 6. The dose of alpha-amanitin injected was controlled by monitoring co-injected fluorescent dye, fura-2.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block M. L., Moody W. J. Changes in sodium, calcium and potassium currents during early embryonic development of the ascidian Boltenia villosa. J Physiol. 1987 Dec;393:619–634. doi: 10.1113/jphysiol.1987.sp016844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet-Meilhac M., Chambon P. Animal DNA-dependent RNA polymerases. 11. Mechanism of the inhibition of RNA polymerases B by amatoxins. Biochim Biophys Acta. 1974 Jun 27;353(2):160–184. doi: 10.1016/0005-2787(74)90182-8. [DOI] [PubMed] [Google Scholar]
- Fukushima Y. Single channel potassium currents of the anomalous rectifier. Nature. 1981 Nov 26;294(5839):368–371. doi: 10.1038/294368a0. [DOI] [PubMed] [Google Scholar]
- Gallin E. K., Sheehy P. A. Differential expression of inward and outward potassium currents in the macrophage-like cell line J774.1. J Physiol. 1985 Dec;369:475–499. doi: 10.1113/jphysiol.1985.sp015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gurdon J. B. Embryonic induction--molecular prospects. Development. 1987 Mar;99(3):285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Takahashi K. Comparison of properties of calcium channels between the differentiated 1-cell embryo and the egg cell of ascidians. J Physiol. 1984 Feb;347:327–344. doi: 10.1113/jphysiol.1984.sp015068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Takahashi K. Development of ionic channels and cell-surface antigens in the cleavage-arrested one-cell embryo of an ascidian. J Physiol. 1987 May;386:113–133. doi: 10.1113/jphysiol.1987.sp016525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka M., Takahashi K. Changes in holding and ion-channel currents during activation of an ascidian egg under voltage clamp. J Physiol. 1982 Feb;323:267–286. doi: 10.1113/jphysiol.1982.sp014072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y. Electrophysiological and immunohistochemical analysis of muscle differentiation in a mouse mesodermal stem cell line. J Physiol. 1991 Oct;442:711–741. doi: 10.1113/jphysiol.1991.sp018816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meedel T. H., Whittaker J. R. Lineage segregation and developmental autonomy in expression of functional muscle acetylcholinesterase mRNA in the ascidian embryo. Dev Biol. 1984 Oct;105(2):479–487. doi: 10.1016/0012-1606(84)90305-1. [DOI] [PubMed] [Google Scholar]
- Meedel T. H., Whittaker J. R. Messenger RNA synthesis during early ascidian development. Dev Biol. 1978 Oct;66(2):410–421. doi: 10.1016/0012-1606(78)90248-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K., Yoshii M. Analysis of non-linearity observed in the current-voltage relation of the tunicate embryo. J Physiol. 1974 Apr;238(1):55–77. doi: 10.1113/jphysiol.1974.sp010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. J., Hagiwara S. Block of inward rectification by intracellular H+ in immature oocytes of the starfish Mediaster aequalis. J Gen Physiol. 1982 Jan;79(1):115–130. doi: 10.1085/jgp.79.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Nishida H., Satoh N. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. I. Up to the eight-cell stage. Dev Biol. 1983 Oct;99(2):382–394. doi: 10.1016/0012-1606(83)90288-9. [DOI] [PubMed] [Google Scholar]
- Nishida H., Satoh N. Determination and regulation in the pigment cell lineage of the ascidian embryo. Dev Biol. 1989 Apr;132(2):355–367. doi: 10.1016/0012-1606(89)90232-7. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado H., Takahashi K. A simple "neural induction" model with two interacting cleavage-arrested ascidian blastomeres. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6197–6201. doi: 10.1073/pnas.85.16.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado H., Takahashi K. Differentiation of membrane excitability in isolated cleavage-arrested blastomeres from early ascidian embryos. J Physiol. 1990 Aug;427:583–602. doi: 10.1113/jphysiol.1990.sp018189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado H., Takahashi K. Induced neural-type differentiation in the cleavage-arrested blastomere isolated from early ascidian embryos. J Physiol. 1990 Aug;427:603–623. doi: 10.1113/jphysiol.1990.sp018190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado H., Takahashi K. Neural differentiation in cleavage-arrested ascidian blastomeres induced by a proteolytic enzyme. J Physiol. 1993 Apr;463:269–290. doi: 10.1113/jphysiol.1993.sp019594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Membrane currents of the tunicate egg under the voltage-clamp condition. J Physiol. 1976 Jan;254(3):607–638. doi: 10.1113/jphysiol.1976.sp011249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y., Shidara M. Changes in sodium channels during neural differentiation in the isolated blastomere of the ascidian embryo. J Physiol. 1990 Dec;431:39–74. doi: 10.1113/jphysiol.1990.sp018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y., Shidara M. Inactivation kinetics of the sodium channel in the egg and the isolated, neurally differentiated blastomere of the ascidian. J Physiol. 1990 Dec;431:75–102. doi: 10.1113/jphysiol.1990.sp018321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M., Okamura Y. Developmental changes in delayed rectifier K+ currents in the muscular- and neural-type blastomere of ascidian embryos. J Physiol. 1991 Nov;443:277–305. doi: 10.1113/jphysiol.1991.sp018834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini L., Block M. L., Moody W. J. Lineage-specific development of calcium currents during embryogenesis. Science. 1988 Dec 16;242(4885):1572–1575. doi: 10.1126/science.2849207. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Hattori K., Weintraub H. Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell. 1989 Jul 14;58(1):171–180. doi: 10.1016/0092-8674(89)90413-3. [DOI] [PubMed] [Google Scholar]
- Tomlinson C. R., Beach R. L., Jeffery W. R. Differential expression of a muscle actin gene in muscle cell lineages of ascidian embryos. Development. 1987 Dec;101(4):751–765. doi: 10.1242/dev.101.4.751. [DOI] [PubMed] [Google Scholar]
- Whittaker J. R. Segregation during ascidian embryogenesis of egg cytoplasmic information for tissue-specific enzyme development. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2096–2100. doi: 10.1073/pnas.70.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]