Abstract
BACKGROUND:
Pulsed field ablation (PFA) is a promising treatment for atrial fibrillation. We report 1-year freedom from atrial arrhythmia outcomes using monopolar PFA delivered through 3 commercial, contact force–sensing focal catheters.
METHODS:
ECLIPSE AF (Safety & Clinical Performance Study of Catheter Ablation With the Centauri System for Patients With Atrial Fibrillation; NCT04523545) was a prospective, single-arm, multicenter study evaluating acute and chronic safety and performance using the CENTAURI system to deliver focal PFA with TactiCath SE, StablePoint, and ThermoCool ST. Patients with paroxysmal or persistent atrial fibrillation underwent pulmonary vein (PV) isolation under deep sedation or general anesthesia and returned for remapping at 90 days to evaluate chronic durability. Freedom from atrial arrhythmia was evaluated continuously through 12 months using standard rhythm monitoring for symptomatic episodes and 24-hour Holter at 6 and 12 months.
RESULTS:
Eighty-two patients (74% male, 51.2% paroxysmal, and 58.5% deep sedation) were treated. PV isolation was achieved in 100% of targeted veins (322/322) with first-pass isolation in 92.2% (297/322). There were 4 primary safety events in 4 patients (4.9%, 4/82); 1 nonembolic stroke due to exacerbated cardiac tamponade secondary to catheter perforation and 3 hemorrhagic vascular access complications. There were no incidences of adverse event fistula, diaphragmatic paralysis, myocardial infarction, pericarditis, thromboembolism, PV stenosis, transient ischemic attack, or death. Eighty patients (98%) underwent remapping. Optimized PFA cohorts 3, 4, and 5 showed per-patient isolation rates of 60%, 73%, and 81% and per-PV isolation rates of 84%, 90%, and 92%, respectively. One-year freedom from atrial arrhythmia was 80.2% (95% CI, 69.7%–87.4%) for the entire patient sample, including 41 patients who underwent repeat focal PFA with the CENTAURI system at remapping.
CONCLUSIONS:
This study demonstrated that optimization of focal PFA with 3 contact force–sensing, solid-tip ablation catheters resulted in the progressive improvement of PV isolation durability at 3-month remapping and high freedom from atrial arrhythmia survival rates, providing a promising focal PFA treatment option integrated with current ablation workflows.
Keywords: atrial fibrillation; electrocardiography, ambulatory; fibrosis; incidence; pulmonary veins
WHAT IS KNOWN?
Pulsed field ablation (PFA) is an emerging energy source for the treatment of atrial fibrillation due to its potentially favorable safety and efficacy profile. However, most current PFA catheters have been designed with limited feedback on electrode-tissue contact, which has been shown to be critical for durable PFA lesion creation.
Few PFA systems have been subjected to a comprehensive assessment of chronic lesion durability via invasive remapping, which has been proven to be critical for PFA system optimization and improved long-term patient outcomes.
WHAT THE STUDY ADDS
The ECLIPSE AF study (Safety & Clinical Performance Study of Catheter Ablation With the Centauri System for Patients With Atrial Fibrillation) demonstrated the ability to design and deliver focal PFA energy safely and effectively with the CENTAURI system through 3 commercially available, contact force–sensing, focal ablation catheters. Invasive remapping was critical to optimizing the PFA waveform and procedural workflow, and an overall 90-day pulmonary vein isolation durability rate of 89% was achieved in the optimized cohorts.
Optimized chronic lesion durability resulted in improved long-term patient outcomes, with an overall freedom from atrial arrhythmia survival rate of 80% in all treated participants. When focusing on the paroxysmal atrial fibrillation patient cohort treated with the optimized focal PFA waveform, the 12-month freedom from atrial arrhythmia survival rate was 93%.
Pulsed field ablation (PFA) using pulsed electric fields (PEF) is a promising alternative to thermal ablation for the treatment of atrial fibrillation (AF). By disrupting the homeostatic state of the cell, the electric field induces cell death and replacement fibrosis, however, without affecting extracellular structures.1,2 Preclinical evaluation of PFA shows its susceptibility to varying tissue planes, making the energy modality ideal for targeting specific tissue without infiltrating extracardiac structures.3,4 The relatively nonthermal nature of PFA also provides a favorable safety profile compared with thermal modalities.5
Recent clinical studies have consistently demonstrated that PFA can be delivered safely and effectively for standard pulmonary vein isolation (PVI) in patients with paroxysmal AF and persistent AF; however, performing invasive remapping has been critical for PFA dose and workflow optimization to ensure that the acute treatment plan was effectively executed and translated to chronic durability and optimal patient ablation outcomes.6–8 While most current PFA platforms are designed to perform anatomically based PVI only with single-shot catheter designs,9,10 the ECLIPSE AF study (Safety & Clinical Performance Study of Catheter Ablation With the Centauri System for Patients With Atrial Fibrillation) showed promising safety and performance results of focal PFA using the CENTAURI system (CardioFocus, Inc) with commercial, contact force–sensing, solid-tip focal ablation catheters for standard point-by-point PVI in patients with paroxysmal and persistent AF.8 These results confirmed the ability to safely create durable focal PFA lesions; however, it was essential to also evaluate the correlation of durable PVI achieved with focal PFA with long-term freedom from atrial arrhythmia outcomes. Therefore, for the first time, we report 12-month freedom of atrial arrhythmia results using this focal PFA system.
Methods
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Trial Design
The ECLIPSE AF study (NCT04523545) was a prospective, multicenter, single-arm study performed at 2 European centers with approval from each center’s ethics committee and corresponding national regulatory agencies and performed under the principles of the Declaration of Helsinki.8 All enrolled patients provided informed consent. The study sample included patients with drug-refractory paroxysmal or persistent AF intended for first-time PVI. The CENTAURI system integrates with standard EP laboratory systems and permits electrophysiologists to conduct standard ablation workflows. The system contains 3 main components: the generator that delivers biphasic, monopolar energy, the connect device that enables connectivity to compatible ablation catheters and corresponding mapping systems, and the cardiac monitor (IVY Biomedical System) that synchronizes PFA to the R-wave. The system was designed to be compatible with 3 ablation catheters and their associated mapping systems: TactiCath SE & EnSite Precision (EnSite), INTELLANAV STABLEPOINT (StablePoint) & RHYTHMIA HDx (RHYTHMIA), and THERMOCOOL SMARTTOUCH (ThermoCool ST) & CARTO3 (CARTO). The complex engineering and product development process, unique to each catheter/mapping system, resulted in sequential integration with the CENTAURI system in the clinical study, starting with TactiCath SE/Ensite, followed by StablePoint/RHYTHMIA, and ThermoCool ST/CARTO.
The primary safety end point was the incidence of predefined system-related and procedure-related serious adverse events (AEs) of interest within 30 days after using the CENTAURI system. The primary performance end points were (1) acute success, defined as PVI achieved with the CENTAURI system during the index procedure assessed by entrance and exit blocks after a 20-minute waiting period, and (2) chronic success, defined as the per-patient and per-PVI rates at 90 (±15) days assessed by high-density remapping and confirmation of entrance and exit blocks. Additional objectives included evaluating freedom of atrial arrhythmia (AF/atrial flutter [AFL]/atrial tachycardia [AT]) through 12 months assessed by 24-hour Holter monitoring at 6 and 12 months, as well as standard rhythm monitoring of symptomatic episodes requiring medical intervention throughout study participation outside the standard 90-day blanking period.
Procedure and Follow-Up
The study index procedure has been previously described.8 The progressive development of the system’s compatibility with commercial catheters and mapping systems resulted in a sequential evaluation of the CENTAURI system paired with either TactiCath SE/EnSite/Advisor HD-Grid, Stable Point/RHYTHMIA/ORION, or ThermoCool ST/CARTO/PENTARAY. Standard wide antral circumferential ablation was performed with a target contact force of ≥5 g, a constant low irrigation rate of 4 mL/min, and sufficient overlap of each focal lesion to achieve adequate depth at, and between, each PFA application (Figure 1).11–14 Acute PVI was assessed via high-density mapping of the pulmonary veins (PVs) while confirming entrance and exit blocks following a 20-minute waiting period.
Figure 1.
Overlapping focal pulsed field ablation (PFA) lesions were applied along the circumference of the pulmonary veins to ensure adequate base lesion depth at each application. In addition, the treatment spacing between each focal lesion was taken into consideration to ensure adequate effective treatment depth between each focal PFA lesion.
Intraprocedural safety assessments included evaluation of ST-segment changes on ECG and incidence of microbubbles on intracardiac echocardiography during each PFA application. Post-procedure safety assessments included phrenic nerve function, the National Institutes of Health Stroke Scale, and esophageal endoscopy. Patients who consented to participate in the cranial magnetic resonance imaging substudy underwent a diffusion weighted imaging + fluid-attenuated inversion recovery cranial magnetic resonance imaging within 7 days pre-procedure and 72 hours post-procedure. Patients with new abnormal findings returned for a 30-day follow-up cranial magnetic resonance imaging.
Treated patients returned for follow-up at 7 days, 30 days, 90 days, 6 months, and 12 months. At the 90-day visit, PV diameters were reassessed via cardiac computed tomography, and invasive high-density remapping was performed to assess PVI durability and iteratively help improve PFA dosing and procedural workflows performed at the index procedure. Any areas of PV reconnection were retreated with optimized PFA dosing and procedural workflows using the CENTAURI system connected to a compatible focal ablation catheter of the investigator’s choice. Rhythm monitoring was performed via standard-of-care monitoring methods for any symptomatic events requiring medical intervention (eg, antiarrhythmic medication, cardioversion, and hospitalization) outside the standard 90-day blanking period. In addition, at the 6- and 12-month visits, 24-hour Holter monitoring was performed to assess for recurrence of atrial arrhythmia. Antiarrhythmic medication was permitted within the 90-day blanking period; however, it was discontinued after the 90-day remapping procedure.
Statistical Analyses
This study was inferentially powered to provide a >90% probability of detecting ≥1 serious device- or procedure-related AEs, assuming that such events have approximately a 5% risk of occurrence. Study safety and performance end points were analyzed on the per-treatment evaluable sample, which consisted of all patients who received complete PVI treatment with the CENTAURI system, who had no major protocol deviations, and for whom follow-up data were available. Descriptive statistics and graphical summaries were used to present study results. For categorical variables, counts, percentages, and, where appropriate, exact 95% CIs for the estimates were calculated. The 1-year freedom from atrial arrhythmia nonprimary objective was evaluated outside of the standard 90-day blanking period using Kaplan-Meier estimates with a 95% CI. The 1-year timepoint was based on the date of the index ablation procedure. The date of the index procedure was defined as t=0, and the first recurrence of atrial arrhythmia was defined as the earliest timepoint between the recorded AE start date (for symptomatic recurrences requiring medical intervention) and the 24-hour Holter assessment target day at the 6- and 12-month visits (6-month visit=day 181 and 12-month visit=day 366) after the 90-day blanking period. If a patient did not have a recurrence of atrial arrhythmia, the patient was right-censored on the day of early termination or the 12-month target study day. Patients left were defined as the number of patients who were at risk at the end of the study. In this long-term analysis, we sought to explore the freedom from AF/AFL/AT across the entire study sample, as well as within various subgroup comparisons, including PFA workflow development cohorts 1 and 2 versus optimized PFA cohorts 3, 4, and 5, paroxsysmal atrial fibrillation (PAF) versus persistent atrial fibrillation (PerAF), and single procedure versus redo procedure.
Results
A total of 84 patients were enrolled in the study, with 82 patients undergoing PVI with the CENTAURI system and included in the evaluable sample. One patient withdrew consent before the index procedure due to scheduling logistics, and one patient was not treated with the study device at the index procedure due to a technical malfunction of the device. Baseline patient characteristics and medical history are provided in Tables 1 and 2 and demonstrate a standard AF population undergoing first-time PVI (aged 61.0±9.05 years, 74.4% male, 51.2% paroxysmal AF, 60.4±9.30% left ventricular ejection fraction, 43.6±4.65-mm left atrium anteroposterior diameter, 59.8% hypertension, 13.4% type 1 or 2 diabetes, 8.5% history of stroke, or transient ischemic attack).
Table 1.
Baseline Demographics and Clinical Characteristics

Table 2.
Baseline Medical History

Procedure Characteristics
Most patients were treated under deep sedation (58.5%, 48/82) versus general anesthesia (41.5%, 34/82). Esophageal temperature probes (CIRCA S-Cath) were used at one investigational center in only the first 2 patients (2.4%, 2/82) during their index procedures. Intracardiac echo was used for 74.4% (61/82) of index procedures.
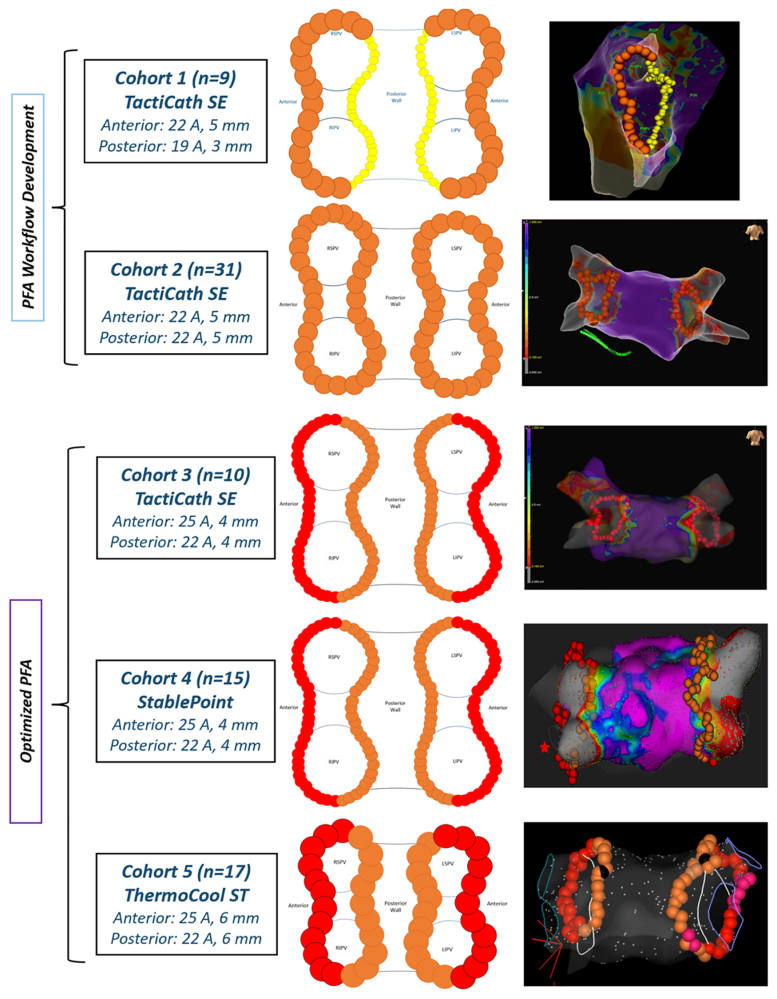
The CENTAURI system was evaluated with solid-tip catheters and their associated mapping systems based on confirmed sequential compatibility. Compatibility was established initially with TactiCath SE/EnSite and evolved to StablePoint/RHYTHMIA and, finally, ThermoCool ST/CARTO. Therefore, treated patients were separated into 5 unique cohorts based on ablation settings, ablation catheter, and mapping system used at the index procedure (Table 3).
Table 3.
Study Patient Cohorts
The energy settings used in each cohort were selected based on anticipated lesion characteristics determined by preclinical models, and dosing and workflow optimization described previously.8,15 Patients in cohort 1 (n=9) and cohort 2 (n=31) were treated with TactiCath SE/EnSite and a part of the PFA workflow development cohorts where the emphasis was put on safety and evaluation of required energy titration per-PVI durability results. Upon optimization of PFA dose and procedural workflows, patients were treated with TactiCath/EnSite in cohort 3 (n=10), StablePoint/RHYTHMIA in cohort 4 (n=15), and ThermoCool ST/CARTO in cohort 5 (n=17), which were considered the optimized PFA cohorts (Figure 2).
Figure 2.
Treated patients were separated into 5 cohorts based on ablation settings, ablation catheter, and mapping system used during the index ablation procedure. LSPV indicates left superior pulmonary vein; PFA, pulsed field ablation; and RPSV, right superior pulmonary vein.
The overall procedure, LA dwell, PFA PVI, and fluoroscopy time for all patients were 140.0±42.44, 117.0±33.14, 61.8±23.03, and 10.3±4.31 minutes, respectively, including the protocol-mandated 20-minute waiting period. For optimized PFA cohort 5, the overall procedure, LA dwell, and PFA PVI time were 137.1±40.42, 112.4±21.55, and 58.5±16.70 minutes, respectively. The number of focal PFA lesions per patient was dependent on the LA size, energy setting, and the lesion tag diameter utilized for each cohort. For all treated patients, regardless of cohort, it took 101.8±31.14 focal lesions to achieve PVI. With the optimized workflow in cohort 5, the number of PFA applications to achieve PVI was reduced to 83.5±19.32.
Safety Results
Acute and chronic safety results (Table 4) have been previously established, and the study achieved its primary safety end point.8 There were 4 primary AEs that met the primary safety end point in 4 patients (4.9%, 4/82), and all were considered related to the standard PVI procedure. There was 1 patient with a nonembolic cerebrovascular accident/stroke due to exacerbated cardiac tamponade secondary to catheter perforation and 3 patients with hemorrhagic events due to vascular access complications. A total of 2 patients experienced cardiac perforation, and 2 patients experienced postop esophagogastroduodenoscopy findings likely due to defective esophageal temperature probes used during the index procedure. There were no incidences of atrioesophageal fistula, diaphragmatic paralysis, myocardial infarction, pericarditis, thromboembolism, PV stenosis, transient ischemic attack, or death. In addition, no late safety signals were observed as there were no reported device- or procedure-related serious AEs during long-term follow-up of patients post-90-day remapping through the 12-month follow-up period.
Table 4.
Study Complications

Clinical Performance Results
Acute procedural success, confirmed by both entrance and exit blocks after a 20-minute waiting period, was achieved in all 82 (100%) treated patients and 322 (100%) PVs, with first-pass isolation in 92.2% (297/322) of treated PVs and 80.5% (66/82) treated patients. Chronic PVI durability was assessed in 98% (80/82) of treated patients at 97.6±25.0 days via invasive high-density remapping. A high-density mapping catheter (Advisor HD-Grid or PENTARAY) was used to circumnavigate the entire circumference of each treated PV to confirm entrance and exit blocks. Overall, the entire evaluated patient sample had a per-patient isolation rate of 51.3% (41/80) and a per-PVI rate of 70.6% (221/313). Patients in the PFA workflow development cohorts 1 and 2 showed a per-patient isolation rate of 38% and 26% and a per-PVI rate of 47% and 53%, respectively (discernably, 5 [62%] and 23 [74%] remapped patients in cohorts 1 and 2 required redo focal PFA with the CENTAURI system, respectively). However, patients in optimized PFA cohorts 3, 4, and 5 showed a per-patient isolation rate of 60%, 73%, and 81% and a per-PVI rate of 84%, 90%, and 92%, respectively (Figure 3; discernably, only 4 [40%], 4 [27%], and 3 [19%] remapped patients in cohorts 3, 4, and 5 required redo focal PFA with the CENTAURI system, respectively). Analysis of the spatial pattern of PV reconnections in the optimized cohorts demonstrated that most focal reconnections were along regions of relatively thicker tissues (eg, superior anterior ridge of left PVs and carinal region of left and right PVs), which are common areas of PV reconnections,16,17 and required a rage of 1 to 3 focal PFA applications to reisolate the PV (Figure S1).
Figure 3.
Chronic pulsed field ablation (PFA) lesion durability was measured per-patient and per-pulmonary vein (PV), at 90 days, for each cohort. Patients in the PFA workflow development cohorts 1 and 2 showed a per-patient isolation rate of 38% and 26% and a per-pulmonary vein isolation rate of 47% and 53%, respectively. Patients in the optimized PFA cohorts 3, 4, and 5 showed a per-patient isolation rate of 60%, 73%, and 81% and a per-PV isolation rate of 84%, 90%, and 92%, respectively. There were no adverse events associated with the optimization in energy settings and clinical workflows.
One-Year Arrhythmia Recurrence
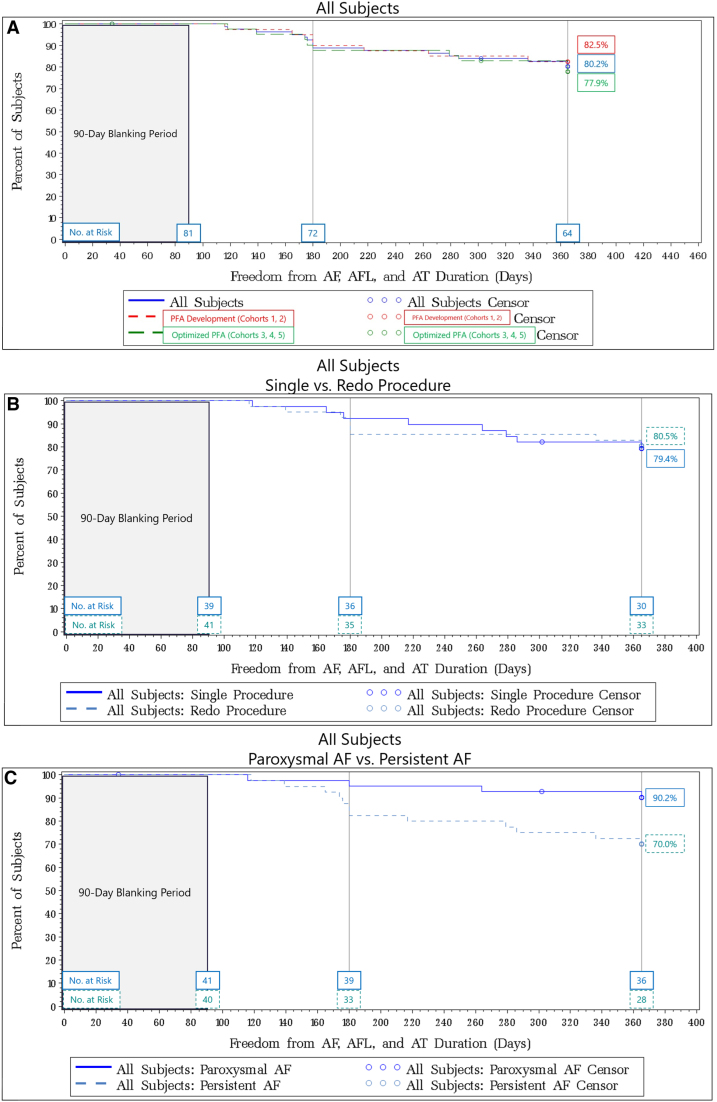
Freedom from atrial arrhythmia (AF, AFL, and AT) was assessed after the 90-day blanking period via standard rhythm monitoring during symptomatic episodes requiring medical intervention and 24-hour Holter monitoring at 6 and 12 months. For the entire patient sample, the 12-month Kaplan-Meier estimate for freedom from atrial arrhythmia was 80.2% (95% CI, 69.7%–87.4%) regardless of cohort, AF classification, or redo PVI at remapping (Figure 4A). The 12-month Kaplan-Meier estimate for PFA development workflow cohorts versus optimized PFA cohorts was 82.5% (95% CI, 66.8%–91.2%) and 77.9% (95% CI, 61.8%–87.8%), respectively.
Figure 4.
Kaplan-Meier curve estimates for freedom of atrial fibrillation (AF)/atrial flutter (AFL)/atrial tachycardia (AT) for all subjects treated in the study. Survival estimates were broken further down by cohort (A), single vs redo procedure (B), and patients with paroxysmal atrial fibrillation (PAF) vs persistent atrial fibrillation (PerAF) (C).
Single procedure versus redo procedure was determined by whether the patient required any additional touch-up PFA at the 90-day remap procedure. For all treated patients, the 12-month Kaplan-Meier estimates for freedom from AF/AFL/AT for patients with single versus redo procedures were 79.4% (95% CI, 63.0%–89.1%) and 80.5% (95% CI, 64.8%–89.7%), respectively (Figure 4B).
When broken down by AF classification for all treated patients, the 12-month Kaplan-Meier estimates for freedom from AF/AFL/AT for patients classified with PAF versus PerAF were 90.2% (95% CI, 75.9%–96.2%) and 70.0% (95% CI, 53.3%–81.7%), respectively (Figure 4C).
Further Kaplan-Meier estimates are provided by cohort, AF classification, and single versus redo procedures in Table S1.
Discussion
The ECLIPSE AF study demonstrated the safety and performance of focal PFA using the CENTAURI system with 3 commercial, contact force–sensing, solid-tip ablation catheters for standard PVI in patients with paroxysmal or persistent AF. A total of 82 patients were treated in the study under deep sedation or general anesthesia, with acute success achieved in 100% of treated patients and first-pass isolation in 92.2% of PVs. There were 4 primary safety events in 4 patients (4.9%), including 1 nonembolic cerebrovascular accident/stroke due to cardiac tamponade secondary to catheter perforation and 3 hemorrhagic events caused by vascular access complications. There was a total of 2 incidences of catheter perforation leading to pericardial effusion requiring pericardiocentesis, an anticipated risk with focal catheter ablation,18,19 and 2 patients with postop esophagogastroduodenoscopy findings that met the definition of a category 1 (mild) esophageal lesion20 and were linked to insulation defects discovered along the thermocouples of the probes utilized during the index procedure. All safety events were deemed related to the PVI procedure.
A critical element of this study was the use of invasive remapping to iteratively determine optimized PFA dosing and clinical workflows and confirm whether the intended treatment plan at the index procedure was effectively executed and resulted in chronically durable lesions. Dosing and procedural workflows were adjusted for each cohort based on the preceding 90-day remap PVI results in the study. The study demonstrated an overall per-PV chronic isolation rate of 70.6% for the entire treated patient sample, which improved to a per-PV chronic isolation rate of 89% within optimized PFA cohorts 3, 4, and 5 with comparable results across all 3 tested ablation catheters and mapping systems. Cohort 5, which presented PFA dosing and workflows most comparable to commercial ablation methods, resulted in a per-patient isolation rate of 81% and a per-PVI rate of 92%. In this cohort, overall procedure (137.1±40.42 minutes), LA dwell (112.4±21.55 minutes), and PFA PVI (58.5±16.70 minutes) times were comparable to times when performing focal RF ablation and even alternative single-shot PFA technologies evaluated in the PULSED AF (Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF; PAF, 134±50 minutes; PerAF, 145±60 minutes) and ADVENT (Randomized Controlled Trial for Pulsed Field Ablation versus Standard of Care Ablation for Paroxysmal Atrial Fibrillation; 105.8±29.4) trials.21–23 However, it should be noted that procedure times included baseline and postablation electroanatomical mapping, as well as a 20-minute waiting period. More importantly, this clinical study was not designed to evaluate the procedural speed and, in lieu, focused on assessing the safety and overall clinical performance of delivering focal PFA with the CENTAURI system. While time intervals were recorded, investigators in this study emphasized taking the necessary time required to ensure safe, and effective focal PFA was delivered for all patients. In addition, dwell times were also dependent on the number of delivered PFA lesions, which, in turn, was dependent on the mapping lesion tag diameter size. The volume of a 3-mm-diameter tag is ≈10× less than a 6-mm-diameter tag and, therefore, results in a greater number of required overlapping lesions to complete a wide antral circumferential ablation PVI, especially in patients with persistent AF with larger left atrial volumes. The necessity of overlapping focal PFA lesions for contiguous, transmural PVI is due to the generally nonthermal formation of ablated tissue with CENTAURI focal PFA. There is no thermal conductivity supporting lesion maturation over time, and focal PFA lesions are likely to be limited to the acute focal imprint generated.13,14
The 12-month Kaplan-Meier estimates for freedom from atrial arrhythmia showed promising results for the overall patient sample at 80.2%. Importantly, all patients who required touch-up ablation to achieve PVI at the 90-day remapping procedure received optimized PFA treatment. Therefore, from the remapping procedure through 12 months, all patients were either confirmed durable or received optimized PFA treatment at remapping to reconfirm PVI durability. This is consistent with the 12-month Kaplan-Meier estimates for freedom from atrial arrhythmia for single versus redo procedures of 79.4% and 80.5%, respectively.
The per-protocol treatment plan involved PVI only, regardless of AF classification. The study enrolled patients without an attempt to balance or control for the distribution of patients with PAF versus PerAF; however, the overall sample was nearly balanced between PAF (42/82, 51%) and PerAF (40/82, 49%). The 12-month Kaplan-Meier estimates for freedom from atrial arrhythmia for subjects classified with PAF versus PerAF were 90.2% versus 70.0%, respectively. Due to the study methods and sequential assessment of PFA dosing and catheter compatibility, subject sampling was not balanced among the various cohorts. As a result, more patients with PerAF (62.5%) were enrolled and treated within the optimized PFA cohorts than in the PFA workflow development cohorts, likely contributing to the slightly lower freedom from atrial arrhythmia survival rates in the optimized PFA cohorts (77.9%) versus the PFA workflow development cohorts (82.5%) because PerAF recurrences are frequently attributed to non-PV triggers.24 It should also be noted that the 95% CIs for these 2 Kaplan-Meier estimates significantly overlap. Looking exclusively at PAF subjects, the 12-month Kaplan-Meier estimate for freedom from atrial arrhythmia for the optimized PFA cohorts was 93.3% (95% CI, 61.3%–99.0%), suggesting that optimized PFA dosing and procedural workflows for a standard PVI-only procedure lead to excellent long-term outcomes for patients with PV-triggered PAF. The 70.0% freedom from atrial arrhythmia rates in the PerAF patient sample is consistent with prior studies and suggests that there is some upper limit of effectiveness for PVI-only ablation strategies in this population. These data suggest that patient-specific ablation strategies beyond PVI will be necessary for greater long-term efficacy and, with optimized focal PFA parameters, should allow investigation of new beyond PVI approached with high confidence that any tested treatment plan was durably executed at the index procedure.
The ECLIPSE AF arrhythmia-free survival results are comparable to the Farapulse IMPULSE/PEFCAT/PEFCAT II 1-year freedom from atrial arrhythmia results for the entire cohort of patients with PAF and optimized biphasic PFA waveform cohort of 78.5±3.8% and 84.5±5.4%, respectively.6 More recently, the MANIFEST-PF registry (Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation), the EU-PORIA registry (European Real World Outcomes with Pulsed Field Ablation in Patients with Symptomatic Atrial Fibrillation), and the ADVENT IDE study showed 1-year freedom from atrial arrhythmia results of 78.1%, 74%, and 73.3%.23,25,26 The Affera Sphere-9 feasibility trial also showed comparable 1-year freedom from atrial arrhythmia results for all treated patients and patients receiving the optimized PULSE3 waveform of 78.1±3.2 and 84.8±4.9%, respectively.7 Notably, these PFA platforms were developed using a protocol-mandated 90-day remapping procedure in their feasibility studies, resulting in PFA dose and workflow optimization. Comparatively, the PULSED AF and InspIRE studies showed overall 1-year freedom from atrial arrhythmia results of 55.1% and 70.9%, respectively, but these technologies did not incorporate invasive 90-day remapping to evaluate lesion durability during their respective feasibility studies.22,27 The incorporation of invasive remapping may aid in efficiently optimizing treatment parameters for acute lesion characteristics and may improve long-term outcomes.
Study Limitations
The ECLIPSE AF study was a single-arm study without a thermal ablation arm for comparison. The sample size was limited within the optimized cohorts 3, 4, and 5 due to the similarity of the 3 solid-tip catheter specifications. Because the performance of the CENTAURI system with compatible focal ablation catheters was evaluated sequentially, the impact of a learning curve on results across the optimized cohorts cannot be ruled out. Weekly/monthly transtelephonic monitoring was not performed as the primary focus of this study was characterizing and optimizing PFA dosing via invasive remapping at 90 days; however, clinically significant recurrences of atrial arrhythmia were captured throughout follow-up for symptomatic episodes requiring medical intervention (eg, antiarrhythmic medication, cardioversion, and hospitalization). Patients who experienced nonsymptomatic arrhythmia recurrence were not seen for any intervention (eg, cardioversion and medication) and, therefore, were not captured as failures in this clinical study when assessing survival from atrial arrhythmia. Due to the sequential development of PFA dose optimization and catheter compatibility, there was no attempt to balance subjects within each cohort based on AF classification, leading to a higher PerAF patient sample in the optimized cohorts than in the initial development cohorts.
Conclusions
In this study, acute and chronic safety and clinical performance of focal PFA were established using the CENTAURI system with 3 commercial, contact force–sensing, solid-tip focal ablation catheters to perform standard PVI in patients classified with paroxysmal and persistent AF. Acute results demonstrated 100% acute PVI with 4 primary safety events occurring in 4 patients (4.9%), and 90-day invasive remapping demonstrated a per-PVI rate of 70.6% for the entire treated patient sample. While the per-PVI rate for the PFA workflow development cohorts 1 and 2 was 52%, the per-PVI rate for the optimized PFA cohorts 3, 4, and 5 was 89%, revealing the necessity to optimize PFA dosing and workflows for improved chronic PVI durability. In turn, this optimization resulted in effective long-term outcomes for treated patients highlighted by the 93.3% freedom from atrial arrhythmia survival rate for patients with PAF treated with optimized PFA, including repeat ablation with focal PFA from the CENTAURI system, if needed, at 3-month remapping. The focal nature of this PFA system enables flexibility to perform beyond PVI workflows that may address the non-PV triggers that possibly contributed to the observed recurrences of atrial arrhythmias in patients with PerAF. Now that treatment parameters and corresponding lesion durability are well established, future studies will investigate the versatility of this focal PFA tool in workflows that incorporate treatment strategies beyond PVI alone. Similar to focal RF ablation technologies that continue to dominate clinical utilization worldwide, a focal PFA platform may help create a full portfolio of ablation tools that can be used in the heterogeneous mix of patients receiving cardiac ablation for a wide range of arrhythmia substrates.
ARTICLE INFORMATION
Acknowledgments
The authors would like to thank Drs Quim Castellvi and Robert Neal, Tim Gundert, and Armaan Vachani for their guidance in developing and optimizing pulsed field ablation with the CENTAURI system. They would also like to thank Drs John C. Evans, Prof Tim R. Betts, and William S. Krimsky for their contributions to medical safety monitoring and interpretation of safety data. They would also like to thank Charlotte Baecke, Julie Bollen, Dr Melda Fabrio, Sophy Yohannan, and Wendy Winters for their commitment to the success of the clinical study. They would also like to thank Ante Borovina, Igor Visković, Sara Milanović, Mirna Žaja, Nela Lemo, Stijn Christiaens, Kim Vandeweyer, Krista Hagfors, and David Gareau for their clinical and technical expertise during support of study procedures.
Sources of Funding
This study was sponsored and funded by CardioFocus, Inc.
Disclosures
Dr Anić serves as a consultant for Galvanize Therapeutics, Boston Scientific, Farapulse, and Biosense Webster; received grant support from Galvanize Therapeutics, Boston Scientific, Farapulse, and Biosense Webster; and owns equity in Agra MedTech, Bolt, and Future Cardia. Drs Vijgen, Philips, and Koopman received grant support from Medtronic, Boston Scientific, Biotronik, Abbott, Pfizer, Bayer, and Daiichi Sankyo. V. Mediratta is an employee of Galvanize Therapeutics. Dr Girouard served as a consultant for Galvanize Therapeutics. The other authors report no conflicts.
Supplemental Material
Table S1
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AE
- adverse event
- AF
- atrial fibrillation
- AFL
- atrial flutter
- AT
- atrial tachycardia
- PFA
- pulsed field ablation
- PV
- pulmonary vein
- PVI
- pulmonary vein isolation
For Sources of Funding and Disclosures, see page 61.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.124.012794.
Contributor Information
Thomas Phlips, Email: thomas.phlips@jessazh.be.
Toni Brešković, Email: toni.breskovic@mefst.hr.
Vikramaditya Mediratta, Email: vikmed95@gmail.com.
Zrinka Jurišić, Email: zrinkacn@gmail.com.
Pieter Koopman, Email: pieter.koopman@jessazh.be.
Nathalie Antole, Email: nathalie.antole@gmail.com.
Johan Vijgen, Email: johan.vijgen@jessazh.be.
References
- 1.Verma A, Asivatham SJ, Deneke T, Castellvi Q, Neal RE. Primer on pulsed electrical field ablation. Circ Arrhythm Electrophysiol. 2021;14:e010086. doi: 10.1161/CIRCEP.121.010086 [DOI] [PubMed] [Google Scholar]
- 2.Sugrue A, Maor E, Del-Carpio Munoz F, Killu AM, Asirvatham SJ. Cardiac ablation with pulsed electric fields: principles and biophysics. Europace. 2022;24:1213–1222. doi: 10.1093/europace/euac033 [DOI] [PubMed] [Google Scholar]
- 3.Neal RE. B-PO05-004 the influence of heterogeneous environments on pulsed electric field therapy effects: implications in cardiac ablation. Heart Rhythm. 2021;18:S372. doi: 10.1016/j.hrthm.2021.06.924 [Google Scholar]
- 4.Moshkovits Y, Grynberg D, Heller E, Maizels L, Maor E. Differential effect of high-frequency electroporation on myocardium vs. non-myocardial tissues. Europace. 2023;25:748–755. doi: 10.1093/europace/euac191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma A, Zhong P, Castellvi Q, Girouard S, Mediratta V, Neal RE. Thermal profiles for focal pulsed electric field ablation. JACC Clin Electrophysiol. 2023;9:1854–1863. doi: 10.1016/j.jacep.2023.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, Cochet H, Minami K, Breskovic T, Sikiric I, et al. Pulsed field ablation of paroxysmal atrial fibrillation. JACC Clin Electrophysiol. 2021;7:614–627. doi: 10.1016/j.jacep.2021.02.014 [DOI] [PubMed] [Google Scholar]
- 7.Reddy VY, Peichl P, Anter E, Rackauskas G, Petru J, Funasako M, Minami K, Koruth J, Natale A, Jais P, et al. A focal ablation catheter toggling between radiofrequency and pulsed field energy to treat atrial fibrillation. JACC Clin Electrophysiol. 2023;9:S2405500X23002190. doi: 10.1016/j.jacep.2023.04.002 [DOI] [PubMed] [Google Scholar]
- 8.Anić A, Phlips T, Brešković T, Koopman P, Girouard S, Mediratta V, Jurisic Z, Sikiric I, Lisica L, Vijgen J. Pulsed field ablation using focal contact force-sensing catheters for treatment of atrial fibrillation: acute and 90-day invasive remapping results. Europace. 2023;25:euad147. doi: 10.1093/europace/euad147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boersma L. New energy sources and technologies for atrial fibrillation catheter ablation. Europace. 2022;24(Supplement_2):ii44–ii51. doi: 10.1093/europace/euab258 [DOI] [PubMed] [Google Scholar]
- 10.Koruth J, Kawamura I, Dukkipati SR, Neuzil P, Reddy VY. Preclinical assessment of the feasibility, safety and lesion durability of a novel ‘single-shot’ pulsed field ablation catheter for pulmonary vein isolation. EP Europace. 2023;25:1369–1378. doi: 10.1093/europace/euad030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa H, Castellvi Q, Neal R, Girouard S, Ikeda A, Kuroda S, Hussein AA, Saliba WI, Wazni OM. B-PO03-131 effects of contact force on lesion size during pulsed field ablation. Heart Rhythm. 2021;18:S242–S243. doi: 10.1016/j.hrthm.2021.06.605 [Google Scholar]
- 12.Taghji P, El Haddad M, Phlips T, Wolf M, Knecht S, Vandekerckhove Y, Tavernier R, Nakagawa H, Duytschaever M. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol. 2018;4:99–108. doi: 10.1016/j.jacep.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa H, Castellvi Q, Neal RE, Girouard S, An Y, Hussein AA, Saliba WI, Wazni OM. PO-710-06 assessment of lesion durability produced by pulsed electric field ablation: evaluation in a swine atrial linear lesion model. Heart Rhythm. 2022;19:S475. doi: 10.1016/j.hrthm.2022.03.1122 [Google Scholar]
- 14.Nakagawa H, Castellvi Q, Neal RE, Girouard S, An Y, Hussein AA, Saliba WI, Wazni OM. PO-710-03 temporal and spatial evolution of reversible and irreversible ventricular lesion boundaries produced by pulsed electric field ablation. Heart Rhythm. 2022;19:S473–S474. doi: 10.1016/j.hrthm.2022.03.1119 [Google Scholar]
- 15.Verma A, Neal R, Evans J, Castellvi Q, Vachani A, Deneke T, Nakagawa H. Characteristics of pulsed electric field cardiac ablation porcine treatment zones with a focal catheter. J Cardiovasc Electrophysiol. 2023;34:99–107. doi: 10.1111/jce.15734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tohoku S, Chun KRJ, Bordignon S, Chen S, Schaack D, Urbanek L, Ebrahimi R, Hirokami J, Fabrizio B, Schmidt B. Findings from repeat ablation using high-density mapping after pulmonary vein isolation with pulsed field ablation. EP Europace. 2023;25:433–440. doi: 10.1093/europace/euac211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohnen M, Weber R, Minners J, Jadidi A, Eichenlaub M, Neumann FJ, Arentz T, Lehrmann H. Characterization of circumferential antral pulmonary vein isolation areas resulting from pulsed-field catheter ablation. Europace. 2023;25:65–73. doi: 10.1093/europace/euac111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798–1803. doi: 10.1016/j.jacc.2009.02.022 [DOI] [PubMed] [Google Scholar]
- 19.Mujović N, Marinković M, Marković N, Kocijančić A, Kovačević V, Simić D, Ristić A, Stanković G, Miličić B, Putnik S, et al. Management and outcome of periprocedural cardiac perforation and tamponade with radiofrequency catheter ablation of cardiac arrhythmias: a single medium-volume center experience. Adv Ther. 2016;33:1782–1796. doi: 10.1007/s12325-016-0402-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller P, Dietrich JW, Halbfass P, Abouarab A, Fochler F, Szöllösi A, Nentwich K, Roos M, Krug J, Schade A, et al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm. 2015;12:1464–1469. doi: 10.1016/j.hrthm.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 21.Kottmaier M, Popa M, Bourier F, Reents T, Cifuentes J, Semmler V, Telishevska M, Otgonbayer U, Koch-Büttner K, Lennerz C, et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. EP Europace. 2020;22:388–393. doi: 10.1093/europace/euz342 [DOI] [PubMed] [Google Scholar]
- 22.Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FE, Calkins H, Sanders P, Packer DL, Kuck KH, et al. ; on behalf of the PULSED AF Investigators. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation. 2023;147:1422–1432. doi: 10.1161/circulationaha.123.063988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, Mountantonakis SE, Gibson DN, Harding JD, Ellis CR, et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med. 2023;389:1660–1671. doi: 10.1056/nejmoa2307291 [DOI] [PubMed] [Google Scholar]
- 24.Yang SY, Cha MJ, Oh HJ, Cho MS, Kim J, Nam GB, C KJ. Role of non-pulmonary vein triggers in persistent atrial fibrillation. Int J Arrhythm. 2023;24:7. doi: 10.1186/s42444-023-00088-0 [Google Scholar]
- 25.Turagam MK, Neuzil P, Schmidt B, Reichlin T, Neven K, Metzner A, Hansen J, Blaauw Y, Maury P, Arentz T, et al. Safety and effectiveness of pulsed field ablation to treat atrial fibrillation: one-year outcomes from the MANIFEST-PF registry. Circulation. 2023;148:35–46. doi: 10.1161/circulationaha.123.064959 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt B, Bordignon S, Neven K, Reichlin T, Blaauw Y, Hansen J, Adelino R, Ouss A, Füting A, Roten L, et al. EUropean real-world outcomes with Pulsed field ablatiOn in patients with symptomatic atRIAl fibrillation: lessons from the multi-centre EU-PORIA registry. Europace. 2023;25:euad185. doi: 10.1093/europace/euad185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duytschaever M, De Potter T, Grimaldi M, Anic A, Vijgen J, Neuzil P, Van Herendael H, Verma A, Skanes A, Scherr D, et al. Paroxysmal AF ablation using a novel variable-loop biphasic pulsed field ablation catheter integrated with a 3D mapping system: 1-year outcomes of the multicenter inspIRE study. Circ Arrhythm Electrophysiol. 2023;16:CIRCEP.122.011780. doi: 10.1161/CIRCEP.122.011780 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.