Abstract
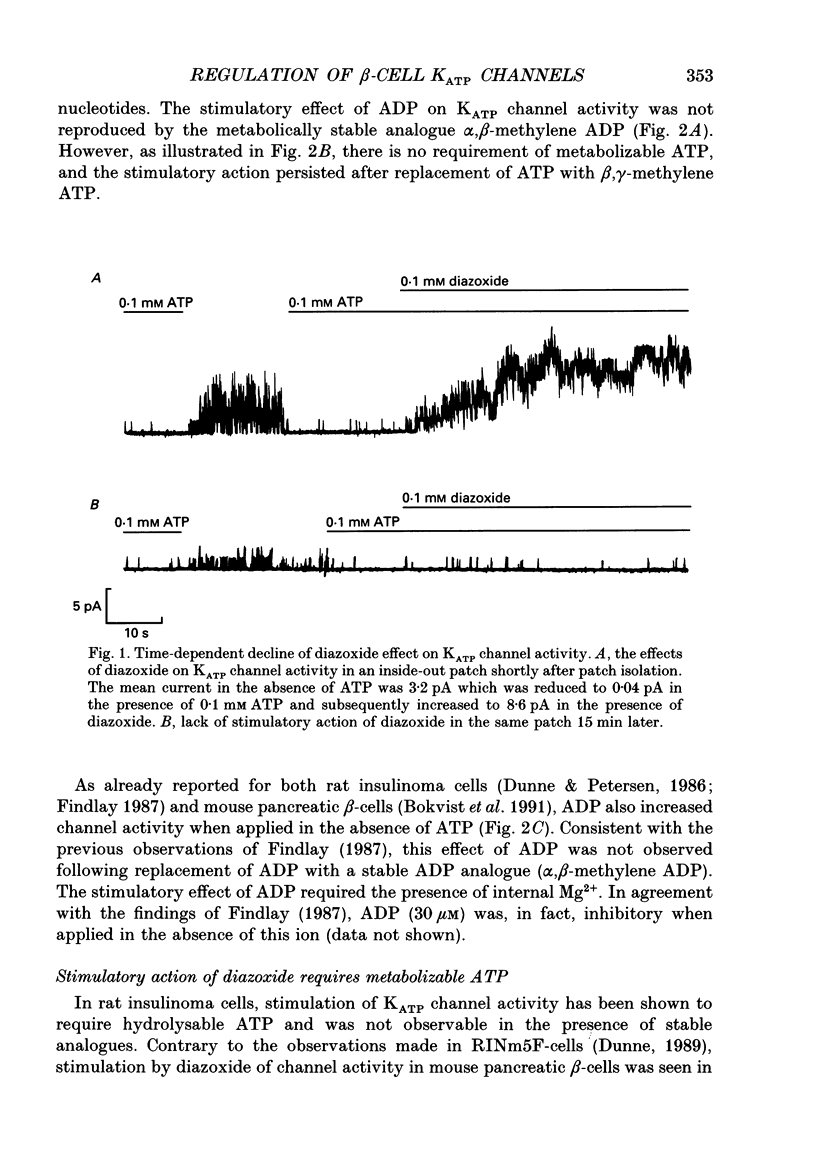
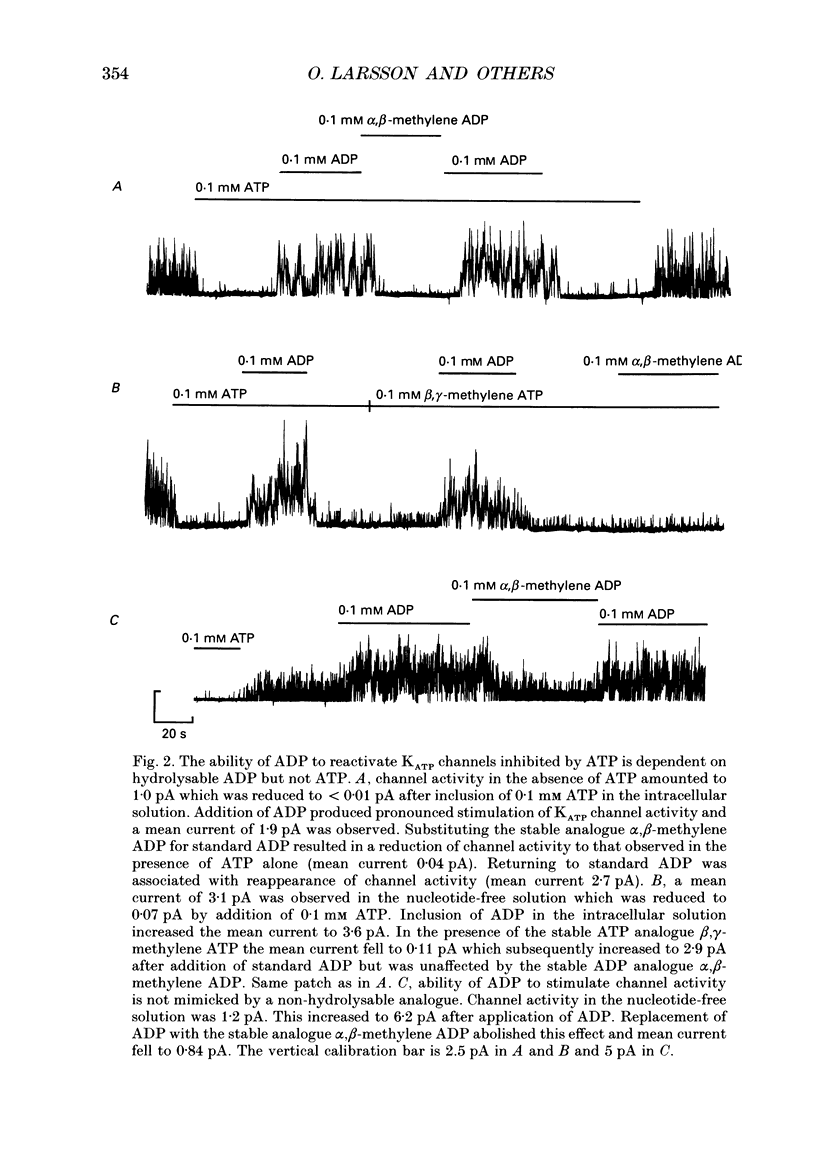
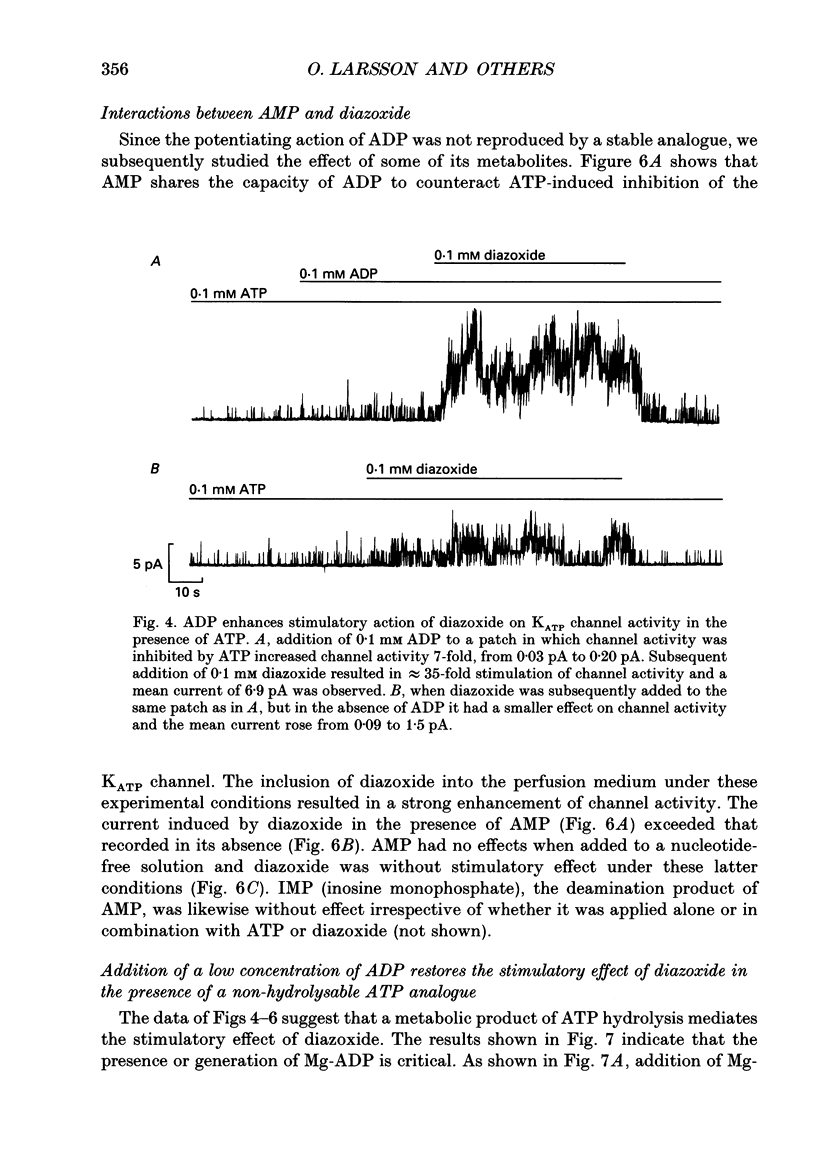
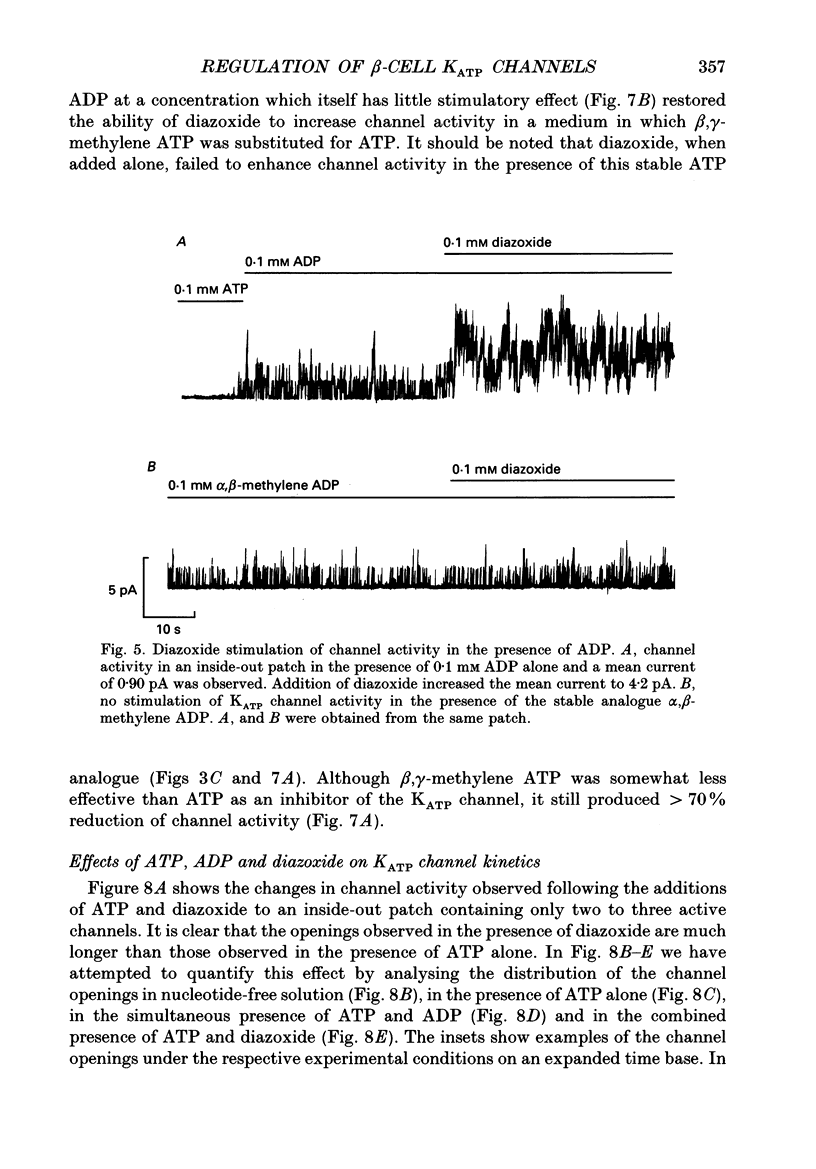
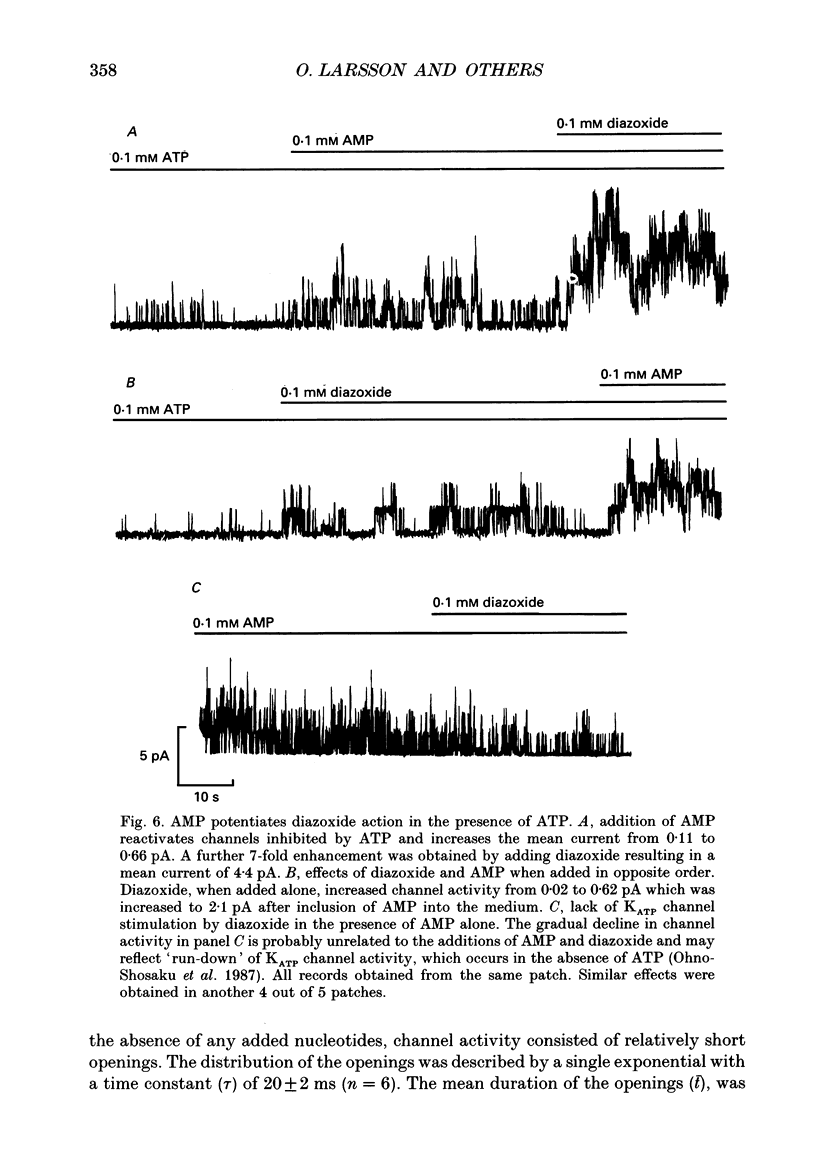
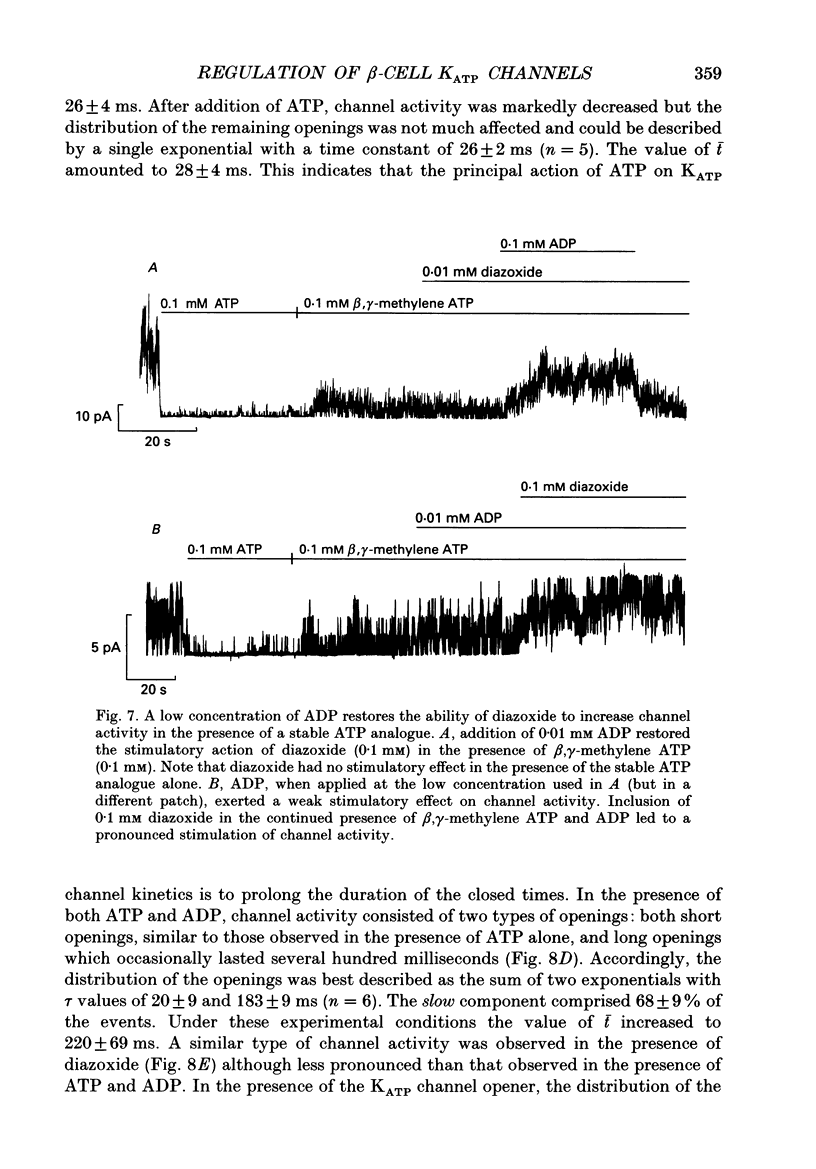
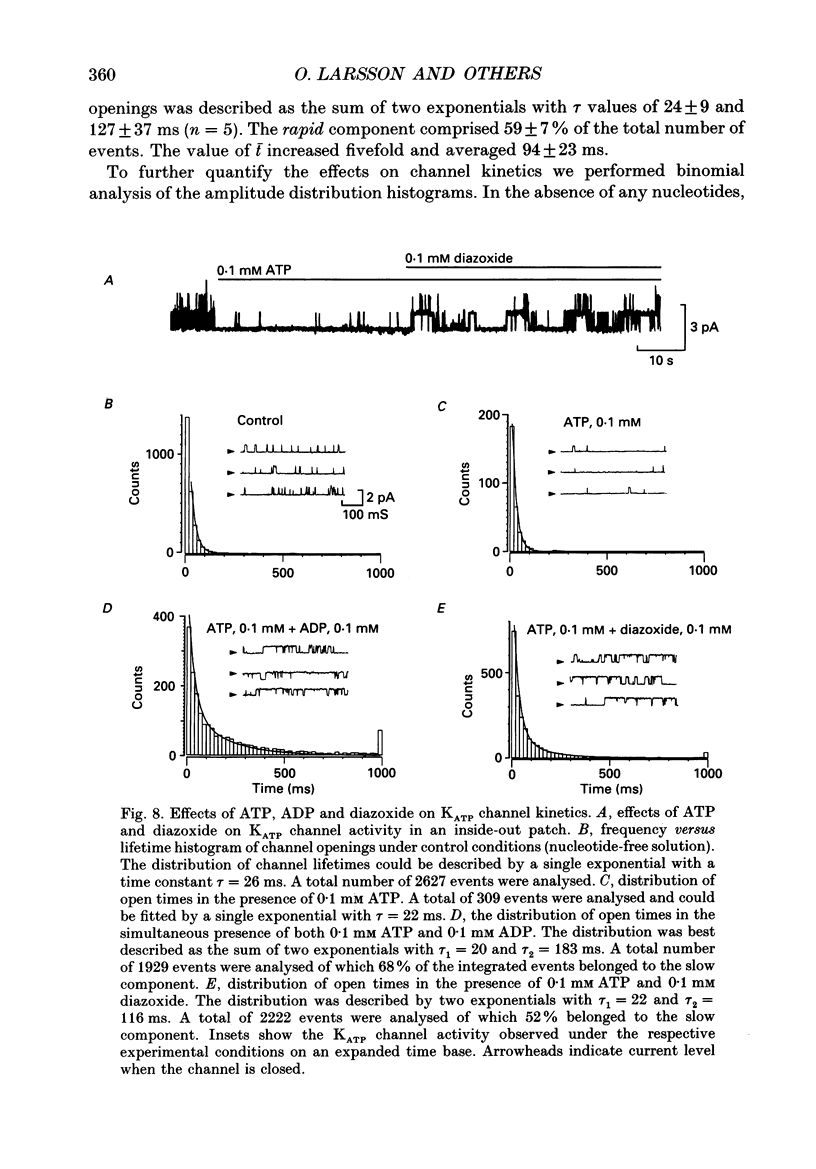
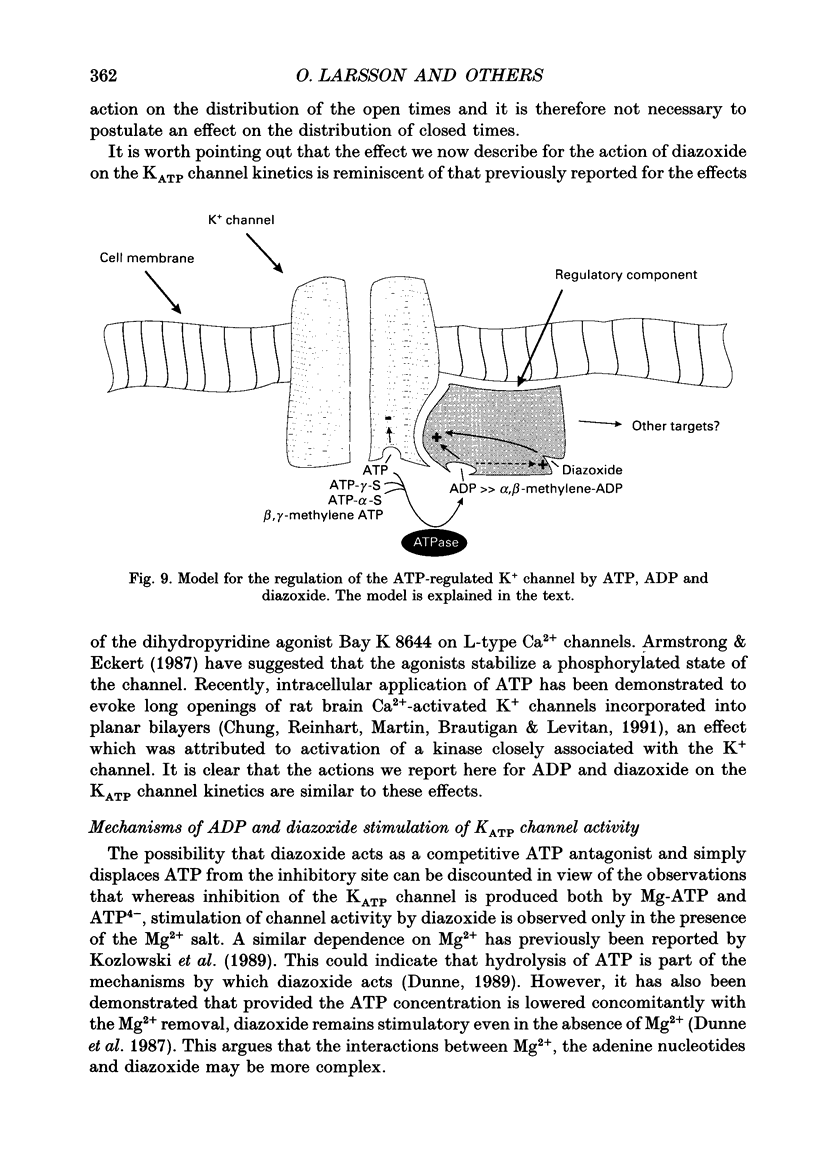
1. The mechanisms by which ADP and the hyperglycaemic compound diazoxide stimulate the activity of the ATP-regulated K+ channel (KATP channel) were studied using inside-out patches isolated from mouse pancreatic beta-cells maintained in tissue culture. 2. The ability of diazoxide and ADP to increase KATP channel activity declined with time following patch excision and no stimulation was observed after 15-40 min. 3. Activation of KATP channels by ADP required the presence of intracellular Mg2+. The stimulatory effect of ADP was mimicked by AMP but only in the presence of ATP. Replacement of ATP with the non-hydrolysable analogue beta, gamma-methylene ATP did not interfere with the ability of ADP to stimulate KATP channel activity. By contrast, enhancement of KATP channel activity was critically dependent on hydrolysable ADP and no stimulation was observed after substitution of alpha,beta-methylene ADP for standard ADP. 4. The ability of diazoxide to enhance KATP channel activity was dependent on the presence of both internal Mg2+ and ATP. Diazoxide stimulation of KATP channel activity was not observed after substitution of beta,gamma-methylene ATP for ATP. However, in the presence of ADP, at a concentration which in itself had no stimulatory action (10 microM), diazoxide was stimulatory also in the presence of the stable ATP analogue. 5. The stimulatory action of diazoxide on KATP channel activity in the presence of ATP was markedly enhanced by intracellular ADP. This potentiating effect of ADP was not reproduced by the stable analogue alpha,beta-methylene ADP and was conditional on the presence of intracellular Mg2+. A similar enhancement of channel activity was also observed with AMP (0.1 mM). In the absence of ATP, diazoxide was still capable of stimulating channel activity provided ADP was present. This effect was not reproduced by AMP. 6. In both nucleotide-free solution and in the presence of 0.1 mM ATP, the distribution of the KATP channel open times were described by a single exponential with a time constant of approximately 20 ms. Addition of ADP or diazoxide resulted in the appearance of a second component with a time constant of > 100 ms which comprised 40-70% of the total number of events. Under the latter experimental conditions, the open probability of the channel increased more than fivefold relative to that observed in the presence of ATP alone.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



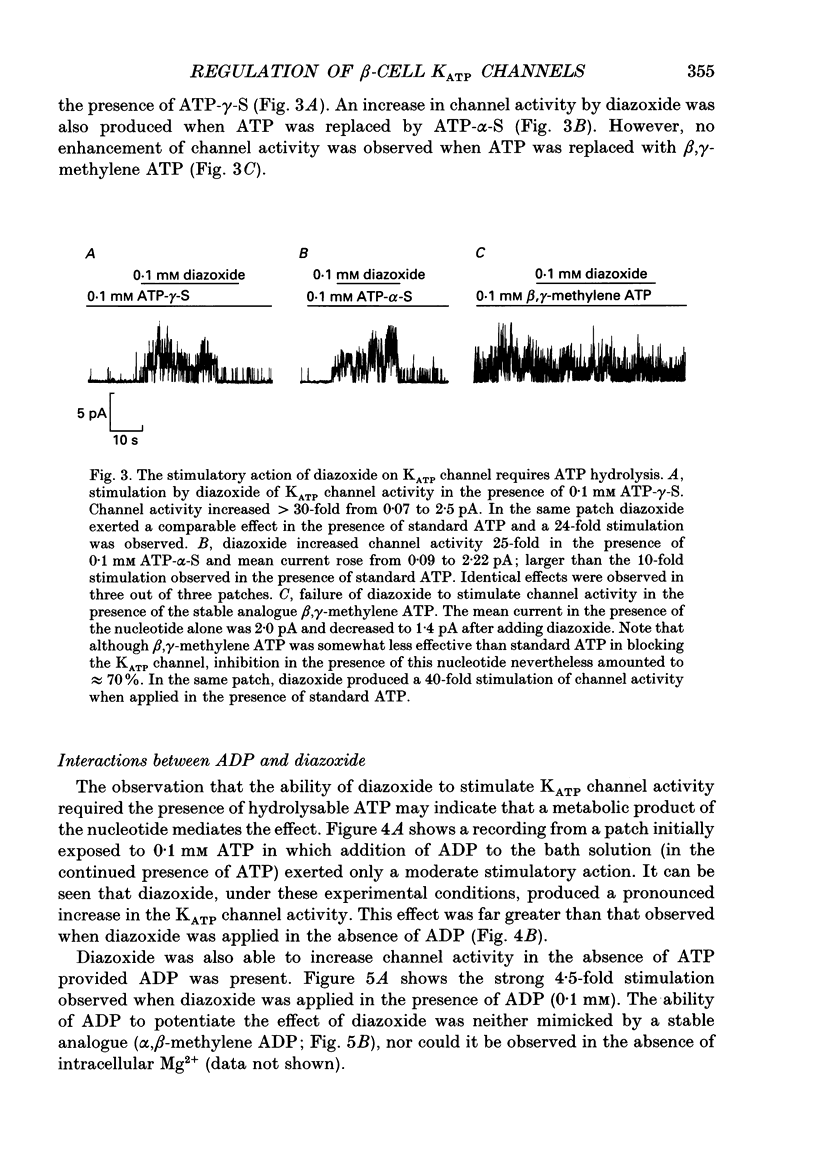




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987 Apr 25;262(12):5448–5454. [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Kakei M. ATP-sensitive K+ channels in rat pancreatic beta-cells: modulation by ATP and Mg2+ ions. J Physiol. 1989 Sep;416:349–367. doi: 10.1113/jphysiol.1989.sp017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Bokvist K., Ammälä C., Ashcroft F. M., Berggren P. O., Larsson O., Rorsman P. Separate processes mediate nucleotide-induced inhibition and stimulation of the ATP-regulated K(+)-channels in mouse pancreatic beta-cells. Proc Biol Sci. 1991 Feb 22;243(1307):139–144. doi: 10.1098/rspb.1991.0022. [DOI] [PubMed] [Google Scholar]
- Chung S. K., Reinhart P. H., Martin B. L., Brautigan D., Levitan I. B. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991 Aug 2;253(5019):560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Illot M. C., Peterson O. H. Interaction of diazoxide, tolbutamide and ATP4- on nucleotide-dependent K+ channels in an insulin-secreting cell line. J Membr Biol. 1987;99(3):215–224. doi: 10.1007/BF01995702. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 1986 Nov 10;208(1):59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- Dunne M. J. Protein phosphorylation is required for diazoxide to open ATP-sensitive potassium channels in insulin (RINm5F) secreting cells. FEBS Lett. 1989 Jul 3;250(2):262–266. doi: 10.1016/0014-5793(89)80734-3. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., West-Jordan J. A., Abraham R. J., Edwards R. H., Petersen O. H. The gating of nucleotide-sensitive K+ channels in insulin-secreting cells can be modulated by changes in the ratio ATP4-/ADP3- and by nonhydrolyzable derivatives of both ATP and ADP. J Membr Biol. 1988 Sep;104(2):165–177. doi: 10.1007/BF01870928. [DOI] [PubMed] [Google Scholar]
- Findlay I. The effects of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J Physiol. 1987 Oct;391:611–629. doi: 10.1113/jphysiol.1987.sp016759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis K. D., Gee W. M., Hammoud A., McDaniel M. L., Falke L. C., Misler S. Effects of sulfonamides on a metabolite-regulated ATPi-sensitive K+ channel in rat pancreatic B-cells. Am J Physiol. 1989 Dec;257(6 Pt 1):C1119–C1127. doi: 10.1152/ajpcell.1989.257.6.C1119. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Charles S., Nenquin M., Mathot F., Tamagawa T. Diazoxide and D600 inhibition of insulin release. Distinct mechanisms explain the specificity for different stimuli. Diabetes. 1982 Sep;31(9):776–783. doi: 10.2337/diab.31.9.776. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986 Nov 10;208(1):63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Hales C. N., Ashford M. L. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. Br J Pharmacol. 1989 Aug;97(4):1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S., Falke L. C., Gillis K., McDaniel M. L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Panten U., Heipel C., Rosenberger F., Scheffer K., Zünkler B. J., Schwanstecher C. Tolbutamide-sensitivity of the adenosine 5'-triphosphate-dependent K+ channel in mouse pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):566–574. doi: 10.1007/BF00169047. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. K., Tung R. T., Machulda M. M., Kurachi Y. Essential role of nucleotide diphosphates in nicorandil-mediated activation of cardiac ATP-sensitive K+ channel. A comparison with pinacidil and lemakalim. Circ Res. 1991 Oct;69(4):1152–1158. doi: 10.1161/01.res.69.4.1152. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Lins S., Ohno-Shosaku T., Trube G., Panten U. Cytosolic ADP enhances the sensitivity to tolbutamide of ATP-dependent K+ channels from pancreatic B-cells. FEBS Lett. 1988 Nov 7;239(2):241–244. doi: 10.1016/0014-5793(88)80925-6. [DOI] [PubMed] [Google Scholar]