Abstract
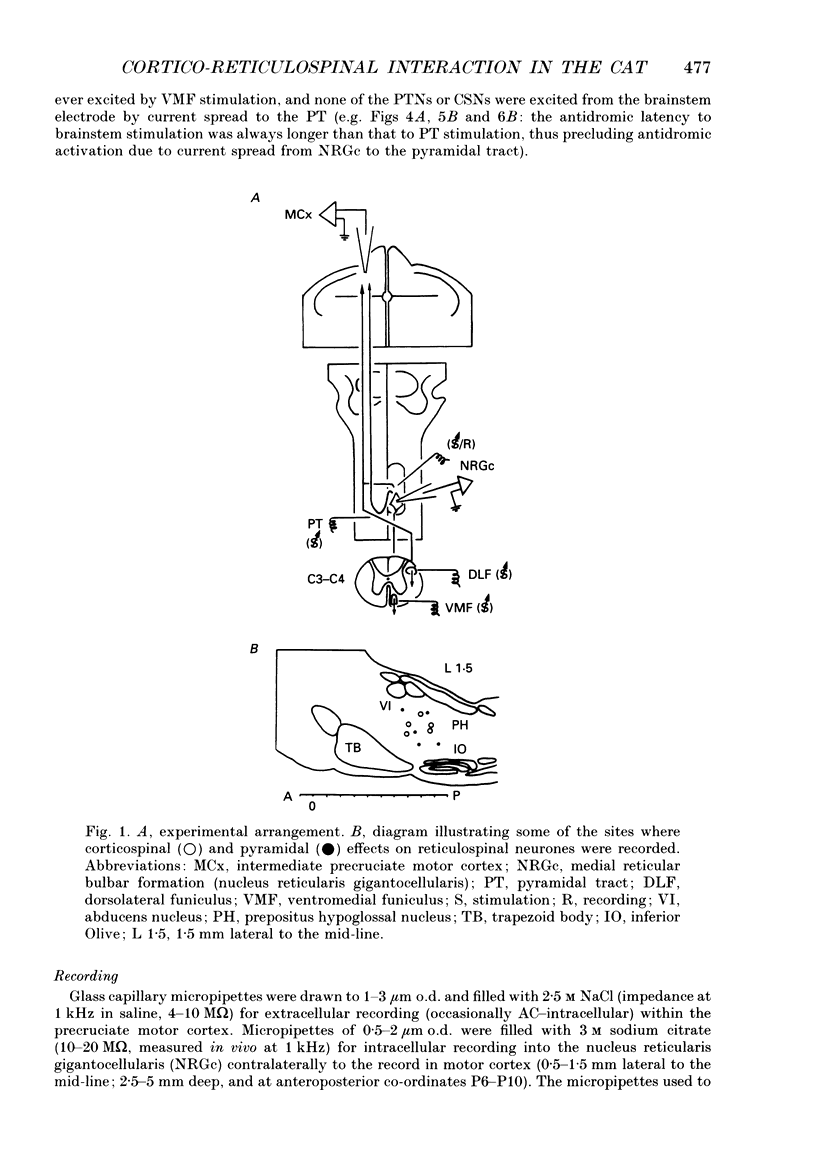
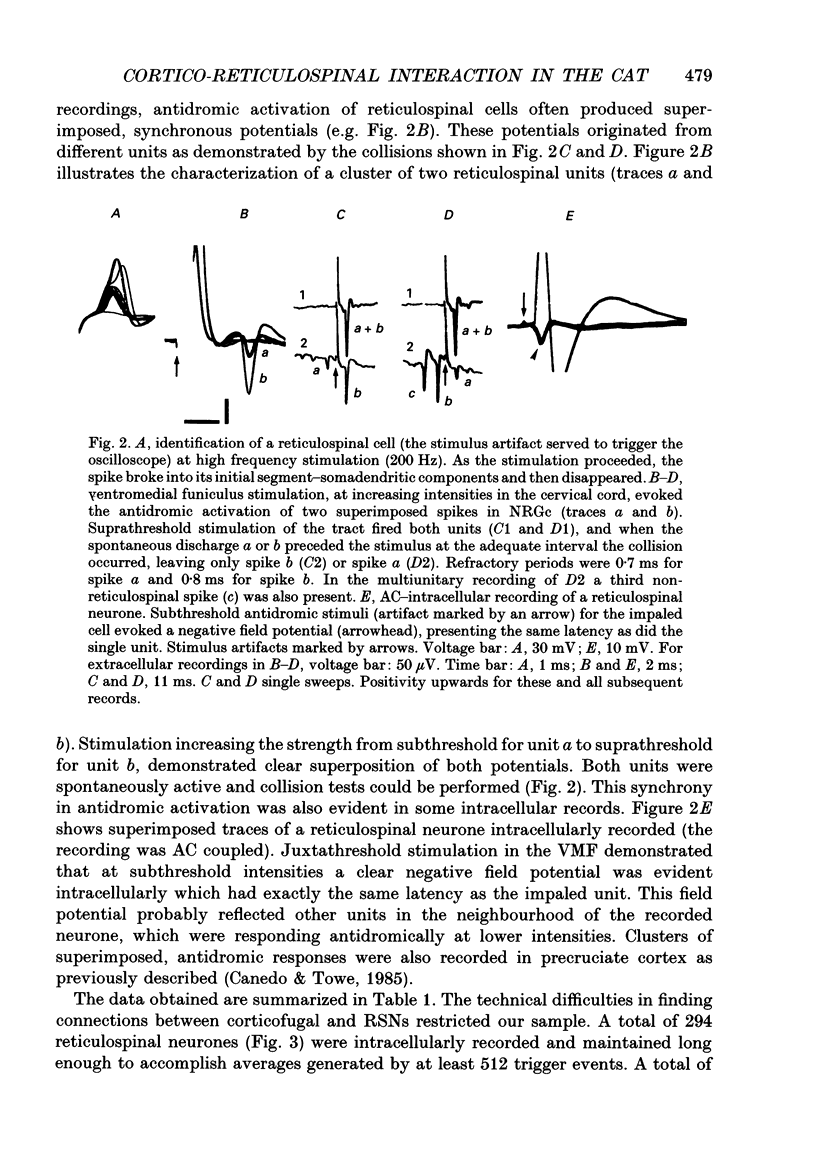
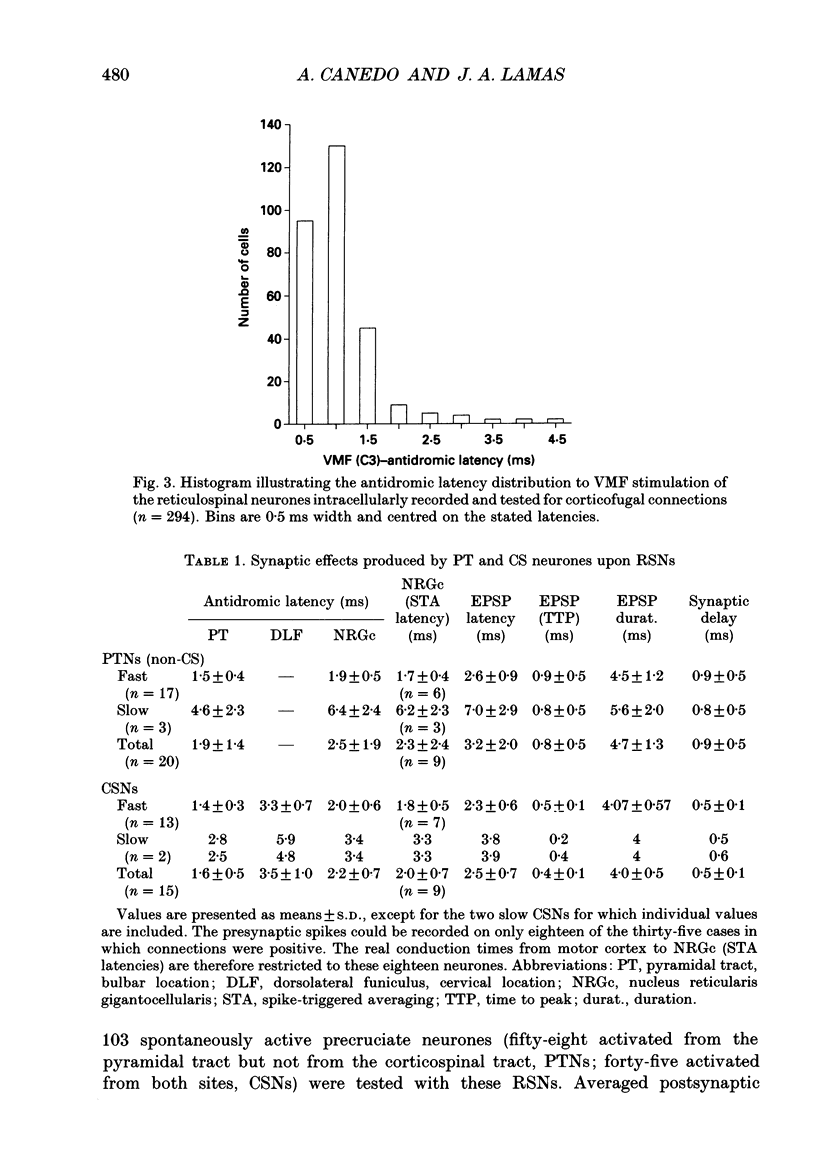
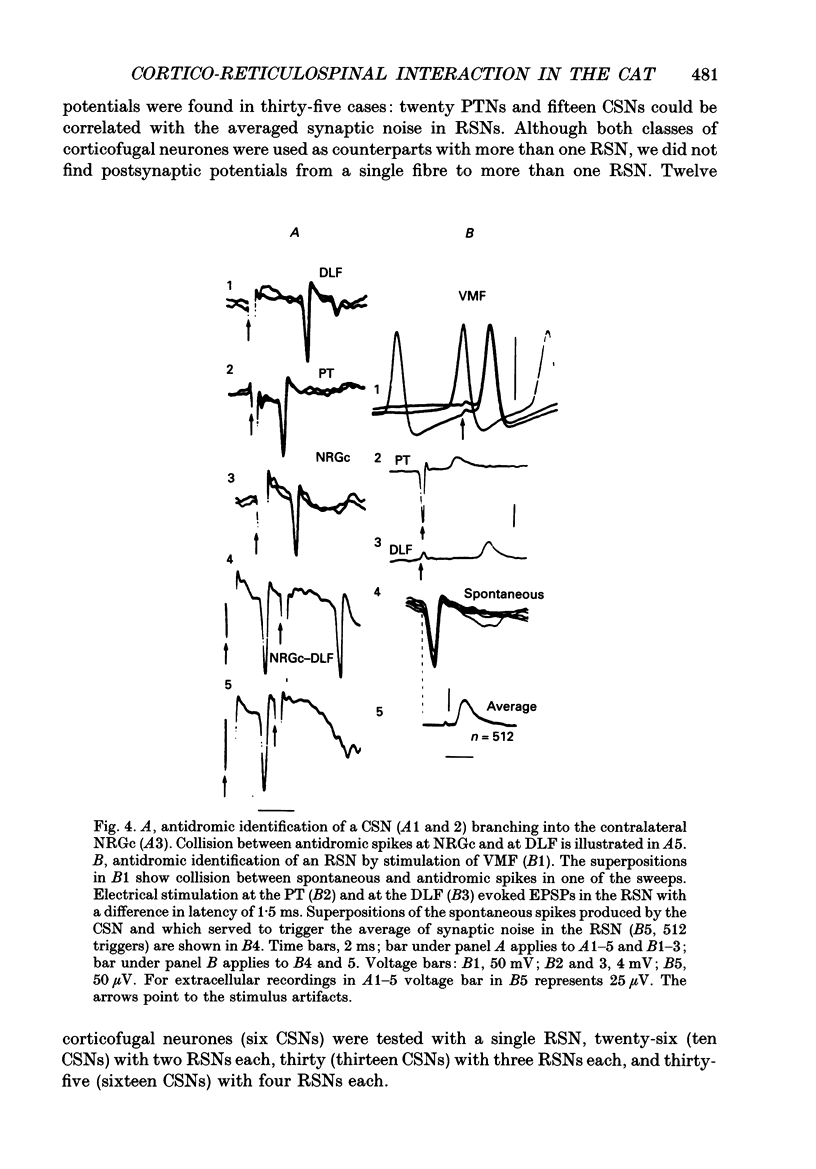
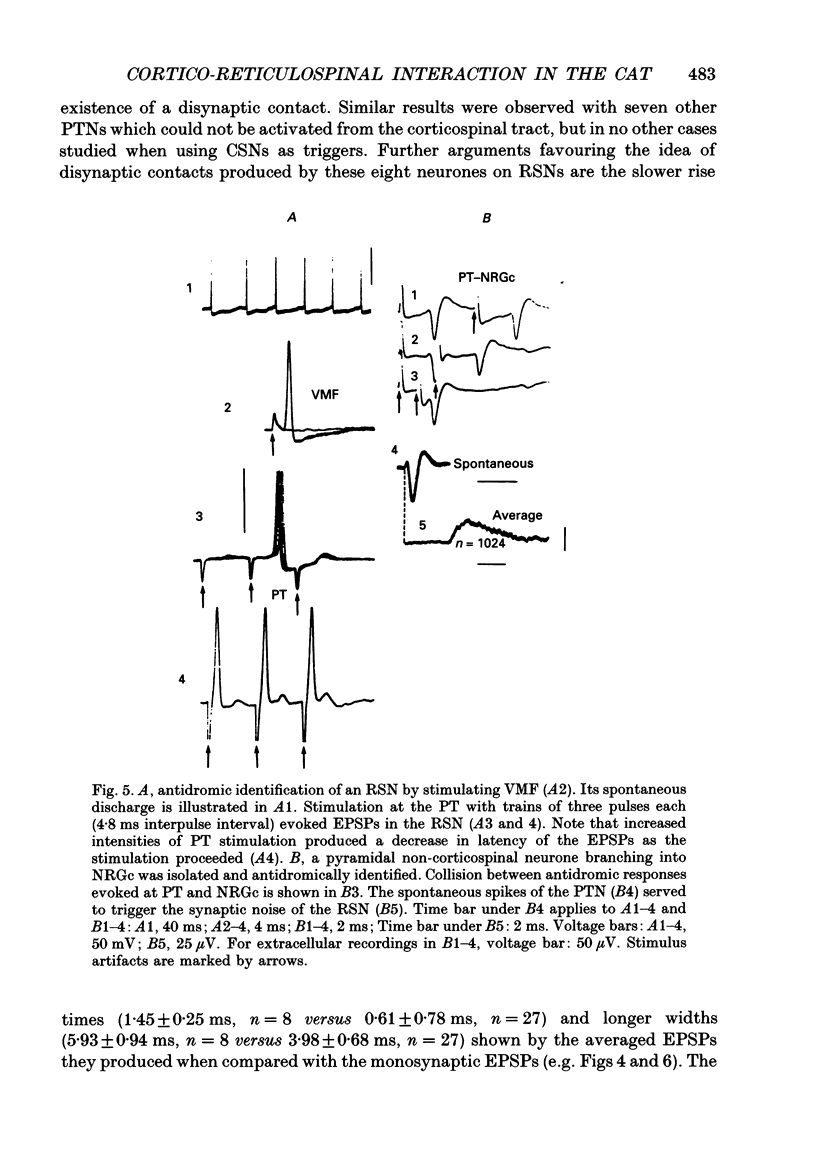
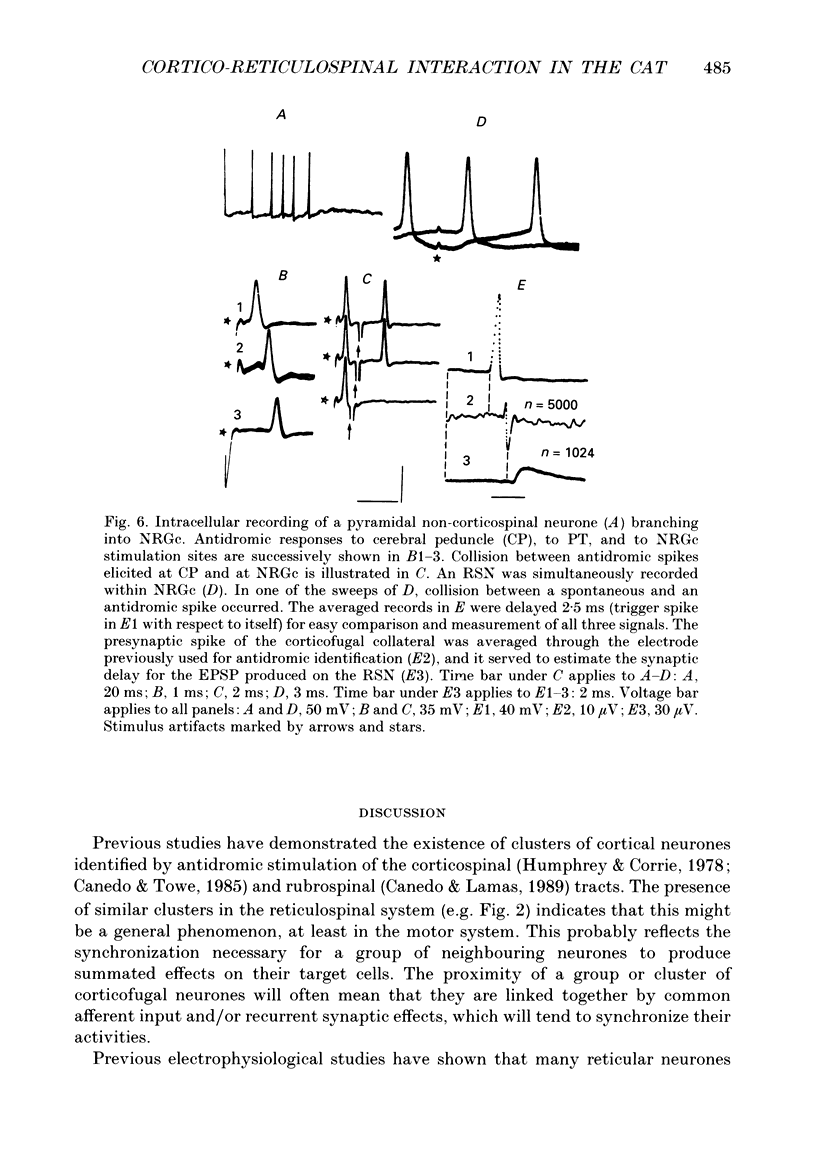
1. The spontaneous activity of 103 precruciate neurones (fifty-eight activated antidromically from the pyramidal tract but not from the corticospinal tract, PTNs; forty-five activated from both sites, CSNs) was used to trigger the average of the intracellularly recorded synaptic noise in 294 reticulospinal neurones (RSNs). These RSNs were recorded in the nucleus reticularis gigantocellularis of the contralateral medial bulbar reticular formation (NRGc) in chloralose-anaesthetized cats. 2. Twelve pyramidal tract neurones (six CSNs) were tested with a single RSN, twenty-six (10 CSNs) with two RSNs each, thirty (13 CSNs) with three RSNs each, and thirty-five (16 CSNs) with four RSNs each. Postsynaptic potentials were observed in the averages generated by twenty PTNs and fifteen CSNs. 3. The only synaptic effect produced by both PTNs and CSNs upon RSNs in our sample was excitatory, and in none of the tested cases (n = 15) were any changes found in the amplitude, shape, or duration of the excitatory postsynaptic potentials (EPSPs) after injection of depolarizing or hyperpolarizing currents. This suggests that the synapses are probably located at the distal dendrites. 4. Recording of the presynaptic spike allowed separation of the conduction time and synaptic delay from the total latency. According to our data there appear to be two different types of excitation of corticofugal neurones over RSNs: a monosynaptic effect produced by both PTNs and CSNs, and a disynaptic effect produced by PTNs but not by CSNs. The disynaptic EPSPs had statistically significant slower rise times and longer widths than the monosynaptic EPSPs.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alstermark B., Pinter M., Sasaki S. Brainstem relay of disynaptic pyramidal EPSPs to neck motoneurons in the cat. Brain Res. 1983 Jan 17;259(1):147–150. doi: 10.1016/0006-8993(83)91078-8. [DOI] [PubMed] [Google Scholar]
- Alstermark B., Pinter M., Sasaki S. Convergence on reticulospinal neurons mediating contralateral pyramidal disynaptic EPSPs to neck motoneurons. Brain Res. 1983 Jan 17;259(1):151–154. doi: 10.1016/0006-8993(83)91079-x. [DOI] [PubMed] [Google Scholar]
- Berthoz A., Grantyn A. Neuronal mechanisms underlying eye-head coordination. Prog Brain Res. 1986;64:325–343. doi: 10.1016/S0079-6123(08)63427-5. [DOI] [PubMed] [Google Scholar]
- Canedo A., Lamas J. A. Rubrospinal tract of the cat: superposition of antidromic responses and changes in axonal excitability following orthodromic activity. Brain Res. 1989 Nov 13;502(1):28–38. doi: 10.1016/0006-8993(89)90458-7. [DOI] [PubMed] [Google Scholar]
- Canedo A., Towe A. L. Pattern of pyramidal tract collateralization to medial thalamus, lateral hypothalamus and red nucleus in the cat. Exp Brain Res. 1986;61(3):585–596. doi: 10.1007/BF00237585. [DOI] [PubMed] [Google Scholar]
- Canedo A., Towe A. L. Superposition of antidromic responses in pyramidal tract cell clusters. Exp Neurol. 1985 Sep;89(3):645–658. doi: 10.1016/0014-4886(85)90014-7. [DOI] [PubMed] [Google Scholar]
- Drew T. Functional organization within the medullary reticular formation of the intact unanesthetized cat. III. Microstimulation during locomotion. J Neurophysiol. 1991 Sep;66(3):919–938. doi: 10.1152/jn.1991.66.3.919. [DOI] [PubMed] [Google Scholar]
- Endo K., Araki T., Yagi N. The distribution and pattern of axon branching of pyramidal tract cells. Brain Res. 1973 Jul 27;57(2):484–491. doi: 10.1016/0006-8993(73)90154-6. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D., German D. C. Corticomotoneuronal connections of precentral cells detected by postspike averages of EMG activity in behaving monkeys. Brain Res. 1976 Sep 24;114(3):505–510. doi: 10.1016/0006-8993(76)90973-2. [DOI] [PubMed] [Google Scholar]
- Grantyn A., Ong-Meang Jacques V., Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. II. Morphological properties as revealed by intra-axonal injections of horseradish peroxidase. Exp Brain Res. 1987;66(2):355–377. doi: 10.1007/BF00243310. [DOI] [PubMed] [Google Scholar]
- He X. W., Wu C. P. Connections between pericruciate cortex and the medullary reticulospinal neurons in cat: an electrophysiological study. Exp Brain Res. 1985;61(1):109–116. doi: 10.1007/BF00235626. [DOI] [PubMed] [Google Scholar]
- Humphrey D. R., Corrie W. S. Properties of pyramidal tract neuron system within a functionally defined subregion of primate motor cortex. J Neurophysiol. 1978 Jan;41(1):216–243. doi: 10.1152/jn.1978.41.1.216. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Sasaki S., Suzuki I. Input-output organization of reticulospinal neurones, with special reference to connexions with dorsal neck motoneurones in the cat. Exp Brain Res. 1990;80(2):260–276. doi: 10.1007/BF00228154. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer K., Kuypers H. G. Distribution of corticospinal neurons with collaterals to lower brain stem reticular formation in cat. Exp Brain Res. 1984;54(1):107–120. doi: 10.1007/BF00235823. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974 Nov 15;252(5480):243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- MAGNI F., WILLIS W. D. CORTICAL CONTROL OF BRAIN STEM RETICULAR NEURONS. Arch Ital Biol. 1964 Jul;102:418–433. [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Peterson B. W., Anderson M. E., Filion M. Responses of ponto-medullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res. 1974;21(1):19–44. doi: 10.1007/BF00234256. [DOI] [PubMed] [Google Scholar]
- Peterson B. W., Franck J. I., Daunton N. G. Changes in responses of medial pontomedullary reticular neurons during repetitive cutaneous, vestibular, cortical, and tectal stimulation. J Neurophysiol. 1976 May;39(3):564–581. doi: 10.1152/jn.1976.39.3.564. [DOI] [PubMed] [Google Scholar]
- Peterson B. W., Maunz R. A., Pitts N. G., Mackel R. G. Patterns of projection and braching of reticulospinal neurons. Exp Brain Res. 1975 Oct 24;23(4):333–351. doi: 10.1007/BF00238019. [DOI] [PubMed] [Google Scholar]
- Shinoda Y., Arnold A. P., Asanuma H. Spinal branching of corticospinal axons in the cat. Exp Brain Res. 1976 Oct 28;26(3):215–234. doi: 10.1007/BF00234928. [DOI] [PubMed] [Google Scholar]
- Shinoda Y., Yamaguchi T., Futami T. Multiple axon collaterals of single corticospinal axons in the cat spinal cord. J Neurophysiol. 1986 Mar;55(3):425–448. doi: 10.1152/jn.1986.55.3.425. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Fuller D. R., Brooks V. B. Collateral pyramidal influences on the corticorubrospinal system. J Neurophysiol. 1968 May;31(3):467–484. doi: 10.1152/jn.1968.31.3.467. [DOI] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]


