Abstract
Cells in the kidney medulla are subject to variable and often extreme osmotic stress during concentration of the urine. Previous studies showed that renal inner medullary epithelial (IME) cells respond to hypertonicity by G2 arrest. The purpose of the present study was to investigate the mechanisms involved in initiation and maintenance of G2 arrest. Rapid initiation of G2 arrest after UV radiation is mediated by p38 kinase. Here we find that p38 kinase is responsible for rapid initiation of the G2 delay in IME cells after the hypertonic stress created by adding NaCl. High NaCl, but not high urea, rapidly initiates G2 arrest. Inhibition of p38 kinase by SB202190 (10 μM) blocks the rapid initiation of this checkpoint both in an immortalized cell line (mIMCD3) and in second-passage IME cells from mouse renal inner medulla. p38 inhibition does not affect exit from G2 arrest. The rapid initiation of G2 arrest is followed by inhibition of cdc2 kinase, which is also prevented by SB202190. To assess the possible protective role of G2 arrest, we measured DNA strand breaks as reflected by immunostaining against phospho-histone H2AX, which becomes phosphorylated on Ser-139 associated with DNA breaks. Abrogation of rapid G2/M checkpoint activation by SB202190 increases the histone H2AX phosphorylation in G2/M cells. We propose that the rapid initiation of G2 delay by p38 kinase after hypertonicity protects the cells by decreasing the level of DNA breaks caused by aberrant mitosis entry.
When urine concentration changes, cells of the kidney inner medulla are subject to changes in extracellular NaCl and urea levels that are potentially damaging. Acute increases of tonicity (e.g., high NaCl) or of urea concentration can cause apoptosis (1, 2), and an acute increase in tonicity has been reported to cause DNA double-strand breaks (3). Over the long term (hours to days), renal medullary cells accumulate organic osmolytes that help maintain cell volume and intracellular ionic strength when tonicity increases and help to counteract the perturbing effects of urea when it increases (reviewed in ref. 4). In the short term (minutes), renal medullary cells respond to acute increases in tonicity by cell cycle arrest (1, 2, 5, 6). In the present study, we examine the molecular mechanism underlying G2 arrest caused by hypertonicity.
The duration of hypertonicity-induced G2 arrest depends on the degree of hypertonicity (2). In mIMCD3 cells, G2 arrest lasts 6 h when osmolality is increased to 500 milliosmol/kg by adding NaCl and 20 h at 650 milliosmol/kg. However, little is known about the molecular mechanisms involved. p53 activity affects G1 and S delays caused by hypertonicity but not G2 delay (6).
p38 kinase is a member of the mitogen-activated protein (MAP) kinase family that is activated by a variety of environmental stresses (reviewed in refs. 7 and 8), including hypertonicity (5, 9–13). p38 is required for fast G2 checkpoint activation after UV radiation (14), and its homologue high osmolality glycerol response (HOG1) participates in hypertonic stress-induced G2 delay in yeast (15).
In the present study, we find that p38 activation is necessary for the rapid activation of G2 arrest after acute hypertonic stress in renal inner medullary epithelial (IME) cells, both in an immortalized cell line, mIMCD3, and in second-passage mouse IME cells (P2mIME). Abrogation of the hypertonicity-induced G2 arrest by the p38 inhibitor SB202190 increases histone H2AX phosphorylation at Ser-139, particularly in cells in S and G2/M. Histone H2AX becomes phosphorylated in association with DNA breaks caused by ionizing radiation (16) and during apoptotic chromatin fragmentation (17), making it a convenient tool for estimation of DNA damage. We suggest that rapid G2 checkpoint activation by p38 after hypertonic stress protects cells from DNA breaks caused by aberrant mitosis entry.
Materials and Methods
Cell Cultures.
mIMCD3 cells.
Subconfluent cultures of mIMCD3 cells (18) (generously provided by S. Gullans, Harvard Medical School, Boston) were used in passages 13–17. The medium contained 45% DME low glucose, 45% Coon's Improved Medium mF-12 (Irvine Scientific), and 10% FBS (Life Technologies, Grand Island, NY). Osmolality of control (isotonic) medium, was 320 milliosmol/kg. Hypertonic medium, prepared by adding NaCl, was substituted for the control medium, as indicated. Cells were incubated at 37°C and gassed with 5% CO2/95% air during growth and all experiments.
Mouse P2mIME cells.
The inner medullas from mouse kidneys were dissected and digested in DMEM/F12 without phenol red (Life Technologies) made hyperosmotic by addition of 80 mM of urea and 130 mM of NaCl (resulting osmolality 640 milliosmol/kg) and supplemented with collagenase B (2 mg/ml, Roche Molecular Biochemicals) and hyaluronidase (0.7 mg/ml, Worthington), as previously described (Z. Zhang, Q. Cai, L. Michea, P. Andrews, N.I.D., G. Rocha, and M.B.B., unpublished work). The renal medullary tissue was incubated for 60 min at 37°C under continuous agitation in a humidified incubator (5% CO2, 95% O2). The resulting suspension was centrifuged at 160 × g for 1 min and washed with hyperosmotic enzyme-free DMEM/F12 medium. The cell pellet was resuspended in hyperosmotic (640 milliosmol/kg) culture medium [50% DMEM low glucose (Irvine Scientific)/50% Coon's Improved F12 (Cellgro, Mediatech, Herndon, VA)/80 mM urea/130 mM NaCl/10 mM Hepes (pH 7.5)/2 mM l-glutamine/10,000 units/ml of penicillin G/10,000 units/ml streptomycin sulfate/50 nM hydrocortisone/5 pM 3,3,5-triiodo-l-thyronine/1 nM sodium selenate/5 mg/liter of transferrin/10% FBS). The cell suspension obtained from two kidneys was plated in a 3.5-cm plastic dish (Corning). After they became confluent (48–72 h), cells were harvested by trypsinization in Ca2+/Mg2+ free hyperosmotic Dulbecco's modified phosphate buffer and seeded in four 3.5-cm dishes (passage 1). Passage 2 cells were used for all experiments. For some experiments, passage 1 cells were transferred to 320 milliosmol/kg medium 48 h before study to provide a more direct comparison to results with mIMCD3 cells that were grown at that osmolality.
Analysis of Mitosis by Immunostaining with Antiphospho-Histone H3 Antibody.
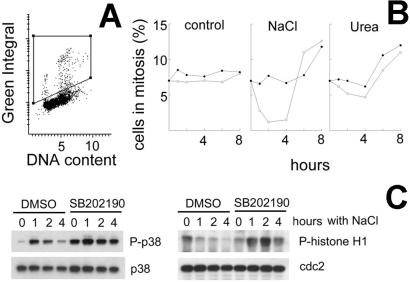
After fixation in 100% methanol at −20°C for 45 min, the cells were washed three times for 5 min each with 0.1% Triton-X100 in PBS, followed by blocking buffer (3% BSA/0.1% Triton-X100). Then they were incubated with antiphospho-histone H3 (mitosis marker) (Upstate Biotechnology, Lake Placid, NY, no. 06–570) antibody, washed with 0.1% Triton-X100 in PBS, incubated for 1 h with secondary antibody (Alexa 488 goat anti-rabbit IgG, Molecular Probes no. A-11034), stained with 0.7 μg/ml of propidium iodide (PI) and mounted with 150 μl of antifade (Molecular Probes, no. S-7461). The slides were analyzed by Laser Scanning Cytometery (CompuCyte, Cambridge, MA). Green fluorescence was recorded to measure antiphospho-histone H3 antibody binding (P-histone H3 content). Red fluorescence was recorded to measure PI binding (DNA content). Bivariate distributions of cells showing P-histone H3 content versus DNA content were obtained, and the percent of P-histone H3 positive (mitotic) cells was determined, as demonstrated in Fig. 2A. The area of the cytograms containing the mitotic cells is bounded by the trapezoid, drawn by eye. This area contains both G1 and G2 cells, because mitotic cells have G2 DNA content before and G1 DNA content after anaphase.
Figure 2.
Inhibition of p38 kinase prevents hypertonicity-induced G2 checkpoint activation. (A) Representative cytogram of mIMCD3 cells at 300 milliosmol/kg, stained for P-H3 (Green Integral) and PI (DNA content). Mitotic cells have high Green Integral fluorescence because of their high level of P-H3. The area of the cytograms containing mitotic cells is bounded by the trapezoid, drawn by eye. Cells falling within this area were classified as mitotic, as confirmed by microscopy. (B) mIMCD3 cells were preincubated for 30 min with vehicle (DMSO, open symbols) or 10 μM p38 inhibitor SB202190 (closed symbols). At 0 time, the medium was replaced to add urea or NaCl to total osmolality 500 milliosmol/kg, as indicated. Cells were fixed and stained with PI for DNA content and with P-H3 antibody for identification of mitotic cells. The percent of mitotic cells was determined by laser-scanning cytometry, as shown in A. (C) mIMCD3 cells were preincubated with SB202190 (10 μM) or DMSO for 30 min, and then the media were changed to otherwise identical ones for 4 h (“0 h with NaCl”) or to an otherwise identical one with NaCl added to total osmolality 550 milliosmol/kg for 1, 2, or 4 h. Protein extracts were prepared, and abundance of cdc2, p38, and p38 phosphorylated on Thr-180/Tyr-182 was determined by Western blot. Also cdc2 was immunoprecipitated, and its kinase activity was measured by phosphorylation of its substrate, histone H1.
Protein Sample Preparation, Immunoprecipitation, Kinase Reactions, Western Blotting, and Immunodetection.
Cells were rinsed with PBS adjusted with NaCl to the same osmolality as the medium, then lysed with lysis buffer [100 mM NaF/50 mM Tris/250 μM thimerosal/1% (vol/vol) Igepal/16 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/5 mM activated NaVO4/protease inhibitors (1 tablet/10 ml, Roche Molecular Biochemicals, no. 1836170)]. After centrifugation for 20 min at 15,000 × g at 4°C, the supernatant was stored at −80°C. Protein content was measured by using the BCA Protein Assay (Pierce). Cdc2 was immunoprecipitated with anti-cdc2 antibody (provided by D. Ferris, National Cancer Institute–National Cancer Research and Development Center) on Protein A Agarose beads (Roche Molecular Biochemicals, no. 1719408). Beads were washed twice with lysis buffer and twice with kinase buffer [25 mM Tris (pH 7.5)/5 mM β-glycerolphosphate/0.1 mM Na3VO4/10 mM MgCl2/2 mM DTT]. Kinase reactions were performed for 30 min at 30°C in 50 μl of the kinase buffer supplemented with 200 μM ATP and 10 μg of the cdc2 substrate Histone H1 (Roche Molecular Biochemicals, no. 223 549). P38 kinase activity was measured by using the p38 MAP Kinase Assay Kit (Cell Signaling, Beverly, MA, no. 9820) according to the manufacturer's instructions. Proteins were separated by SDS/PAGE. Immunodetection used specific antibodies against p38, phospho-p38 (Thr-180/Tyr-182), phospho-ATF2 (Thr-71) (Cell Signaling, nos. 9210, 9211, and 9221) and phospho Histone H1 (Upstate Biotechnology, no. 06-597).
Analysis of Histone H2AX Phosphorylation by Immunostaining with Antiphospho-Histone H2AX Antibodies.
The immunostaining procedure was the same as for antiphospho-histone H3, except that the primary antibody was antiphospho-H2AX (Ser-139) (Upstate Biotechnology, no. 07–164). By using a laser-scanning cytometer, the cells were analyzed for green fluorescence to measure antiphospho-histone H2AX antibody binding and red fluorescence to measure PI binding (DNA content). Bivariate distributions of cells showing maximal intensity of green fluorescence in a nucleus (Green Max Pixel) versus DNA content were obtained. The limits of the region of the cytogram-containing cells with high of Green Max Pixel (phospho-H2AX positive cells) were determined by eye on the basis of control sample (as shown in Fig. 6B), and the percent of cells in that region (phospho-H2AX positive cells) was determined for the G1, S, and G2 phases of the cell cycle.
Statistical Analysis.
The results are presented as representative experiments or as mean ± SEM (n = 4). The statistical significance of differences was determined by ANOVA Parametric Repeated Test, followed by Student–Newman–Keuls Test.
Results
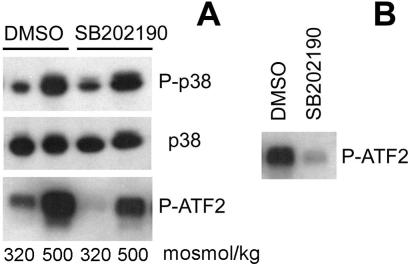
Hypertonicity has previously been reported to increase p38 activity (5, 9–12). To confirm this, we measured p38 abundance, phosphorylation state, and activity. Within 30 min, increasing NaCl to a total osmolality of 500 milliosmol/kg greatly increases phosphorylation and kinase activity of p38 (Fig. 1), consistent with its activation by MAP kinase (MAPK) kinase (7). p38 abundance does not change (Fig. 1). Further, SB202190 inhibits p38 activity without affecting its phosphorylation, consistent with the known mechanism of its action, namely binding to the ATP site in p38 (19). The degree of inhibition is higher when SB202190 is added directly to the kinase reaction (Fig. 1B) than when it is added to the medium bathing the cells (Fig. 1A), because it washes out during immunoprecipitation.
Figure 1.
Effect of the p38 inhibitor, SB202190, on p38 abundance, phosphorylation and activity. (A) mIMCD3 cells were preincubated with SB202190 (10 μM) or DMSO for 30 min, then the media were changed for 30 min to identical ones or to otherwise identical ones with NaCl added to a total osmolality of 500 milliosmol/kg. Western analysis of p38 abundance, phosphorylation, and kinase activity. p38, total p38 protein abundance; P-p38, abundance of p38 phosphorylated on Thr-180/Tyr-182; P-ATF2, phosphorylation of the p38 substrate ATF2, as a measure of p38 kinase activity. (B) p38, activated by increasing NaCl to raise osmolality to 500 milliosmol/kg for 30 min, was immunoprecipitated as described in Materials and Methods, and p38 kinase activity was measured. SB202190 (10 μM) or DMSO was added to the kinase reaction. SB202190 decreases P-ATF2, confirming that it inhibits p38 kinase activity directly.
We used SB202190 to address the potential role of p38 kinase in hypertonicity-induced G2 arrest. G2 checkpoint activation was assessed by measurement of mitotic index. NaCl added to a total osmolality of 500 (Fig. 2B) or 550 (Fig. 3A) milliosmol/kg, rapidly activates the G2 checkpoint, greatly reducing the percent of mitotic cells 1–4 h after NaCl is added. After 4 h, the effect reverses, and the percent of mitotic cells exceeds the control level (Fig. 2B). The decrease in mitosis after elevation of the NaCl level is completely prevented by SB202190, implicating the p38 activation in the process. High NaCl increases p38 phosphorylation during the first 2 h of its application, and the phosphorylation falls close to the basal level after 4 h (Fig. 2C). This time course matches that of the G2 checkpoint activation (Fig. 2A).
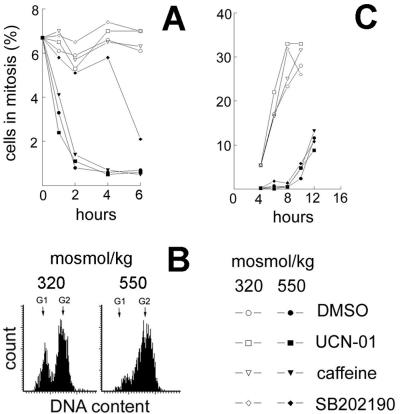
Figure 3.
Effect of SB202190 (p38 inhibitor, 10 μM), UCN-01 (Chk1 inhibitor, 500 nM), or caffeine (ATM, ATR inhibitor, 2 mM) on G2 checkpoint activation and G2 exit. (A) After preincubation with DMSO or inhibitors for 30 min, the medium was changed: NaCl was added to total osmolality of 550 milliosmol/kg, as indicated. Percent of cells in mitosis was determined as in Fig. 1. (B and C) Cells were synchronized in early S by incubation for 11 h with 1 μM of the DNA polymerase inhibitor aphidicolin. Then the media were changed to media free of aphidicolin and made hypertonic by addition of NaCl to a total osmolality of 550 milliosmol/kg, as indicated. After 4 h, when G2 arrest was complete at 550 milliosmol/kg, inhibitors or DMSO were added together with nocodazole (a microtubule inhibitor, 0.5 μM) to trap all cells that entered mitosis. (B) Representative cytograms showing the position of cells in the cell cycle at the time inhibitors were added (4 h). (C) Percent of cells in mitosis, based on P-H3 staining at the indicated time points.
Onset of mitosis is regulated by the activation of Cdc2 kinase (20). After UV radiation, p38 mediates initiation of G2 arrest by phosphorylation of Cdc25B phosphatase, which is the upstream regulator of Cdc2 activity (14). Therefore, we studied the effect of inhibiting p38 activity with SB202190 on Cdc2 activity (Fig. 2C). High NaCl decreases Cdc2 activity as measured by phosphorylation of its substrate histone H1, and inhibition of p38 completely blocks this effect. In fact, Cdc2 activity actually increases during the first hours of hypertonicity if p38 is inhibited (Fig. 2C), suggesting premature mitosis entry. We infer that p38 activates the G2 checkpoint by inhibiting Cdc2. High urea does not activate the G2 checkpoint (Fig. 2B). It was previously observed that urea does not activate p38 (10), which may explain why high urea does not cause rapid G2 arrest.
Other signaling proteins, such as ATM/ATR and Chk1, are also known to regulate the G2 checkpoint after DNA damage (21–24). However, as shown in Fig. 3A, inhibitors of these signaling proteins, caffeine (ATM/ATR) (25) and UCN-01 (Chk1) (26), do not affect initiation of the NaCl-induced G2 checkpoint. Thus, p38 is involved in initiation of G2 arrest after high NaCl, but ATM, ATR, and Chk1 are not. We examined maintenance of the NaCl-induced G2 arrest and recovery from it by synchronizing the cells with the reversible DNA polymerase inhibitor, aphidicolin, and by adding the microtubule inhibitor, nocodazole, to eliminate exit from mitosis. As seen in Fig. 2, when NaCl is added to 500 milliosmol/kg, G2 arrest develops within 1 h and ends after 4 h, as cells begin to enter mitosis again. The duration of G2 arrest after acute hypertonic stress depends on how much the osmolality is increased (2). To accentuate G2 arrest, we increased NaCl further to a total osmolality of 550 milliosmol/kg. As shown in Fig. 3C, inhibition of p38 (by SB202190), of ATM/ATR (by caffeine), or of Chk1 (by UCN-01) does not affect the time course of the exit from G2 arrest into mitosis. Further, none of these inhibitors affects G2/M progression at 320 milliosmol/kg (Fig. 3C). Thus, p38 activity is necessary for initiation of the G2 arrest induced by high NaCl, but p38 activity does not affect maintenance of the G2 arrest or exit from it.
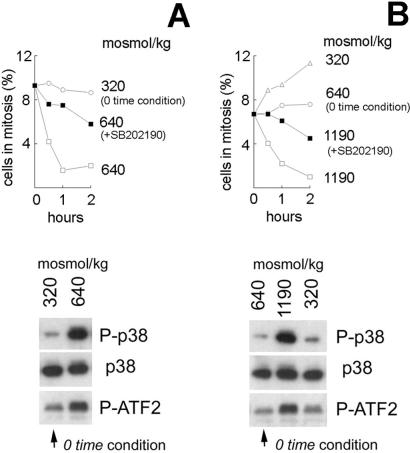
mIMCD3 cells express simian virus 40 constitutively, which immortalizes them. Because of concern that this might affect their response to hypertonic stress, we repeated some of the above studies in P2mIME cells. The interstitial osmolality in inner medullas normally exceeds 600 milliosmol/kg. P2mIME cells grow readily in medium whose osmolality is elevated to 640 milliosmol/kg by adding NaCl and urea, and they readily withstand acute changes in osmolality above and below that baseline. With P2mIME cells (Fig. 4A), similar to the result with mIMCD3 cells, elevating osmolality from 320 to 640 milliosmol/kg by adding NaCl causes G2 arrest associated with activation of p38. Inhibition of p38 by SB202190 greatly reduces G2 arrest. Further, raising osmolality from 640 to 1,190 milliosmol/kg by adding NaCl also activates p38 and causes G2 arrest that is diminished by SB202190 (Fig. 4B). Decreasing osmolality from 640 milliosmol/kg to 320 milliosmol/kg by lowering NaCl has little effect on p38 activity and does not activate G2 checkpoint. In fact, the mitotic index increases (Fig. 4B).
Figure 4.
p38 activation by hypertonicity causes G2 arrest in P2mIME cells. Cells from mouse inner medullas were grown at 640 milliosmol/kg as described in Materials and Methods. Before addition of NaCl, the medium either was changed to 320 milliosmol/kg for 48 h to be more directly comparable to the experiments with mIMCD3 cells or was kept at 640 milliosmol/kg, which is more physiological for these cells. At the time of the experiments, the cells were preincubated with SB202190 (10 μM) or DMSO for 30 min, and then the media were changed to media at the same osmolality or with increased NaCl, as designated, but still containing the same DMSO or SB202190. (A and B Upper) Cells were fixed at indicated times and stained with P-H3 antibody for identification of mitotic cells. The percent of mitotic cells was determined by laser-scanning cytometry. (A and B Lower) Protein extracts were prepared after 30 min of elevated osmolality. p38 was analyzed by Western blot (p38, total p38 protein abundance; P-p38, phosphorylated on Thr-180/Tyr-182). p38 kinase was immunoprecipitated, and its activity is measured by phosphorylation of its substrate ATF2.
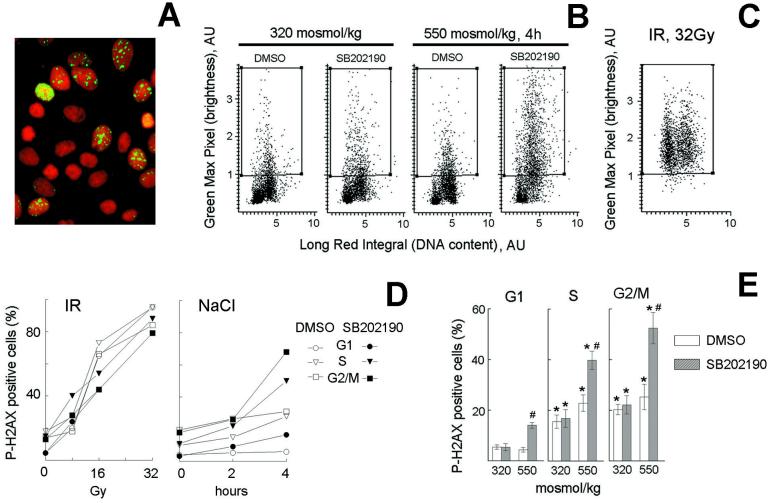
We next tested the effect on histone H2AX phosphorylation of inhibiting G2 arrest with SB202190. Histone H2AX becomes phosphorylated on Ser-139 within seconds after DNA breaks caused either by ionizing radiation (16) or during apoptotic chromatin fragmentation (17). As shown in Fig. 5, inhibition of p38 by SB202190 during hypertonicity is followed by an increase in P-H2AX-positive cells in all phases of cell cycle but especially in S and G2. The proportion of P-H2AX-positive cells increases with time, reaching 52 ± 6% in G2/M cells by 4 h (Fig. 5 D and E). This level of phosphorylation is comparable to that caused by 16 Gy of ionizing radiation (Fig. 5D). However, in contrast to ionizing radiation, which damages cells to the same extent in all phases of the cell cycle (Fig. 5 C and D), damage after p38 inhibition during hypertonicity is much higher outside G1 and is maximal in G2 cells (Fig. 5 B and E). The fact that in the presence of SB202190 the percentage of cells showing DNA damage (P-H2AX positive) is much higher than the percentage of mitotic cells suggests premature aberrant mitosis entry could be occurring. Thus, p38-mediated rapid activation of G2 arrest after hypertonic stress protects cells from DNA damage that would otherwise occur because of aberrant entry into mitosis.
Figure 5.
Abrogation of G2 checkpoint by p38 inhibition induces DNA damage with a cell cycle distribution different from that produced by ionizing radiation (IR). mIMCD3 cells were preincubated with DMSO or SB202190 (10 μM) for 30 min, then, for osmotic stress, the media were replaced for 2 or 4 h with identical ones or with otherwise identical ones to which NaCl was added to total osmolality of 550 milliosmol/kg; for ionizing radiation, cells were irradiated with indicated levels, then returned to the incubator for 20 min. Cells were stained with PI for DNA content (red) and with antiphospho-H2AX (green) to detect DNA breaks. (A) Images of cells stained with antiphospho-H2AX and PI, illustrating localized nuclear antiphospho-H2AX staining when NaCl is added in presence of SB202190. (B and C) Representative cytograms plotting maximal green fluorescence intensity (phospho-H2AX) in the nucleus vs. red fluorescence integral (DNA content). (D and E) G1, S, and G2/M cells were identified, on the basis of their DNA content and the percent of cells in each phase of cell cycle containing localized bright green nuclear fluorescence, illustrated in A, was determined by their elevated maximal green fluorescence intensity (i.e., cells in the boxes in B and C). (D) Representative experiments. (E) Analysis of H2AX phosphorylation in G1, S, and G2/M after 4 h of elevated NaCl (mean ± SEM, n = 6). *, significantly different from G1 (P < 0.01). #, significantly different from control with DMSO (P < 0.01).
Discussion
Exposure of renal IMCD cells to hypertonic stress causes G2 arrest (1, 2, 5, 6). In the present studies, we demonstrate that, similar to the response to UV radiation (14), rapid activation of the G2 arrest after increasing NaCl depends on activation of the p38 MAPK. Further, by using histone H2AX phosphorylation on Ser-139 to measure DNA damage (16, 17), we find that reduction of G2 arrest by inhibiting p38 kinase increases H2AX phosphorylation in S and G2/M. Thus, activation of G2 arrest by p38 protects cells from the DNA damage that would otherwise occur if the cells continued their movement through cell cycle. G2 arrest is a general reaction of cells to environmental stresses. It occurs after ionizing radiation, UV radiation, increased reactive oxygen species, genotoxic chemicals, and heat shock (reviewed in refs. 27 and 28). G2 arrest is believed to provide time for DNA repair and other adaptations to the stress.
p38 kinase was first cloned from murine pre-B cell, on the basis of its rapid tyrosine phosphorylation in response to lipopolysaccharide, and was identified by sequence similarity with yeast MAPK HOG1 gene as a member of the MAPK family (9, 29). At about the same time, p38 was cloned by other groups as a MAPK that is stimulated by IL-1 (30) and sodium arsenite or heat shock (31). Useful piridinyl-imidazole inhibitors of p38 followed from the cloning of p38 as a target of these compounds, which were known as anti-inflammatory drugs inhibiting production of IL-1 and tumor necrosis factor from stimulated human monocytes (32). These compounds have become important tools for studying the roles of p38 (19). The family of p38 kinases includes p38α, p38β, p38γ, and p38δ. P38α and p38β are strongly inhibited by SB202190, whereas the other two isoforms are not (19). p38α and p38β are expressed in numerous tissues (33), and p38α gene disruption is lethal (34). Close to 100 genes are regulated through p38 signaling pathways. Members of the p38 family are involved in numerous cellular processes, including differentiation, inflammation, cell cycle regulation, and cell death (reviewed in refs. 7 and 8).
p38 and Hypertonic Stress.
Yeast HOG1 MAPK mutants have increased susceptibility to hypertonic stress (9, 29). Hypertonicity inhibits growth of these mutant cells more than it does wild-type yeast cells. Wild-type yeast accumulates glycerol, a protective organic osmolyte, in response to hypertonicity, and glycerol accumulation is reduced in HOG1 mutants (35). Mammalian p38 complements the yeast HOG1 mutation, restoring growth in high NaCl medium (9). p38 is activated by hypertonicity in many mammalian cell types, including macrophages (9, 12), renal inner medullary cells (5, 10), myocytes (11), and HepG2 liver cells (13). Renal inner medullary cells are exposed to high levels of NaCl and urea, and those levels are changed associated with changes in urine concentration. Acute elevation of NaCl, but not of urea, activates p38 in mIMCD3 cells (5, 10), and in vivo water restriction increases p38 activity in the renal medulla, where NaCl concentration increases, but not in the renal cortex, where it does not (36, 37). Mammalian cells also accumulate protective organic osmolytes in response to hypertonicity. This helps normalize turgor pressure and intracellular salt concentration (reviewed in refs. 4 and 38). This process takes several hours, and several studies have shown that it might depend on p38 (13, 39–41). To survive during this period, cells arrest their growth.
Hypertonicity delays the cell cycle both in yeast (15) and mammalian cells (1, 2, 5, 6). mIMCD3 cells respond to hypertonic stress by activation of G1, S, and G2 checkpoints. Activation of G1 and S checkpoints depends on p53 activity, which is increased by hypertonicity, but activation of the G2 checkpoint does not (6). p38 is necessary for the rapid activation of G2 arrest after UV radiation (14), and HOG1 participates in hypertonic stress-induced G2 delay in yeast (15). In the present study, we show that rapid activation of the G2 checkpoint in response to hypertonicity is mediated by p38 kinase, similar to the response to UV radiation. It is of interest that, although p38 is necessary for initiation of G2 arrest, it is not necessary for maintenance of the arrest. Inhibition of p38 in cells arrested in G2 by hypertonicity does not accelerate release of cells into mitosis (Fig. 3). Evidently, G2 arrest is maintained by other mechanisms, consistent with the short duration of p38 activation, which ends well before the cells exit from G2 (Fig. 4). In the regulation of G2 arrest and possibly also organic osmolyte accumulation, p38 may act as a “switch” that turns on to activate the process/program and turns off after the process/program is properly started. How p38 senses that the program has been properly started is an interesting question. There is recent evidence for a feedback system. The dephosphorylation that inactivates p38 apparently is an active process that involves a particular phosphatase (42). Our results are consistent with this idea. When p38 is inhibited by SB202190 so that the rapid G2 arrest is prevented (Figs. 2 and 3), the level of p38 phosphorylation does not return to the control level by 4 h as it does when the inhibitor is absent (Fig. 2). Similarly, yeast mutants that are unable to produce glycerol rapidly in response to hypertonicity because of deletion of glycerol 3-P phosphatase use an alternate slower way of synthesizing glycerol. In these mutants, p38 activation is prolonged, and osmosensitivity is increased (43).
Our results demonstrate the advantage of the speed of G2 checkpoint activation by p38. When p38 is inhibited, G2 arrest occurs only after 4 h, compared with 1 h in uninhibited cells (Fig. 3A). By 4 h, the level of histone P-H2AX has increased dramatically (Fig. 5). Thus, rapid, p38-dependent activation of the G2 checkpoint may be important for minimizing DNA damage. Intriguingly, there normally are significant differences in H2AX phosphorylation levels throughout the cell cycle. As shown on Fig. 5, the level of H2AX phosphorylation is significantly higher in S and G2/M, regardless of the osmolality. In the isotonic condition, this is most likely caused by gaps and breaks that occur during DNA replication and perhaps by “errors” in the replication process. Previously, we found a similar cell cycle distribution of p53 phosphorylation on Ser-15 both with and without hypertonic stress (6). Because p53 becomes phosphorylated on Ser-15 after DNA damage (44), this is consistent with a higher level of damage in S and G2/M, just as is the greater H2AX phosphorylation in S and G2/M (Fig. 5). Previously, we found that the rate of DNA synthesis increases dramatically when the P-p53 (Ser-15) level is decreased by caffeine (6). This implies that an S phase checkpoint associated with DNA synthesis is active even under control conditions. Perhaps ATM/ATR–p53 participates in monitoring the progression of normal DNA replication and inhibits replication when stress occurs that could damage replicating DNA. After inhibition of p38, the level of H2AX phosphorylation increases dramatically not only in G2/M but also in S (Fig. 5), suggesting that other effects besides abrogation of G2 arrest may be involved. One possibility is deregulation of the S phase checkpoint that is normally activated very rapidly after hypertonic stress (6). Another possible effect of inhibiting p38 is premature mitosis entry, which is supported by the increased Cdc2 activity shown in Fig. 2C.
Fig. 5 also illustrates a striking difference between the cell cycle distribution of H2AX phosphorylation caused by ionizing radiation and that caused by hypertonic stress. Phosphorylation increases throughout the cell cycle after ionizing radiation, whereas the predominant phosphorylation is in S and G2/M after hypertonicity and increases dramatically when p38 is inhibited. Taken together with our previous finding that hypertonicity kills cells predominantly during the S and G2 phases of the cell cycle, especially when p53 function is impaired (6), these results suggest possible application of hypertonic stress in cancer therapy.
In summary, we propose that p38 is responsible for the rapid activation of G2 arrest after acute hypertonic stress, and that this arrest protects cells from DNA damage that would otherwise occur if the cells continued to enter mitosis.
Acknowledgments
We are very grateful to D. Ferris for the cdc2 polyclonal antibody, to Q. Cai for help with primary cultures preparation, and to D. Kültz for helpful discussions.
Abbreviations
- MAP
mitogen-activated protein
- MAPK
MAP kinase
- IME
renal inner medullary epithelial
- P2mIME
second-passage mouse IME cells
- PI
propidium iodide
- HOG1
high osmolality glycerol
References
- 1.Santos B C, Chevaile A, Hebert M J, Zagajeski J, Gullans S R. Am J Physiol. 1998;274:F1167–F1173. doi: 10.1152/ajprenal.1998.274.6.F1167. [DOI] [PubMed] [Google Scholar]
- 2.Michea L, Ferguson D R, Peters E M, Andrews P M, Kirby M R, Burg M B. Am J Physiol Renal Physiol. 2000;278:F209–F218. doi: 10.1152/ajprenal.2000.278.2.F209. [DOI] [PubMed] [Google Scholar]
- 3.Kültz D, Chakravarty D. Proc Natl Acad Sci USA. 2001;98:1999–2004. doi: 10.1073/pnas.98.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burg M B, Kwon E D, Kültz D. Annu Rev Physiol. 1997;59:437–455. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- 5.Kültz D, Madhany S, Burg M B. J Biol Chem. 1998;273:13645–13651. doi: 10.1074/jbc.273.22.13645. [DOI] [PubMed] [Google Scholar]
- 6.Dmitrieva N, Michea L, Burg M. Am J Physiol Renal Physiol. 2001;281:F522–F530. doi: 10.1152/ajprenal.2001.281.3.F522. [DOI] [PubMed] [Google Scholar]
- 7.Ono K, Han J. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 8.Kyriakis J M, Avruch J. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 9.Han J, Lee J D, Bibbs L, Ulevitch R J. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 10.Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero G N. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 11.Clerk A, Michael A, Sugden P H. J Cell Biol. 1998;142:523–535. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H T, Yang D D, Wysk M, Gatti E, Mellman I, Davis R J, Flavell R A. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadkarni V, Gabbay K H, Bohren K M, Sheikh-Hamad D. J Biol Chem. 1999;274:20185–20190. doi: 10.1074/jbc.274.29.20185. [DOI] [PubMed] [Google Scholar]
- 14.Bulavin D V, Higashimoto Y, Popoff I J, Gaarde W A, Basrur V, Potapova O, Appella E, Fornace A J., Jr Nature (London) 2001;411:102–107. doi: 10.1038/35075107. [DOI] [PubMed] [Google Scholar]
- 15.Alexander M R, Tyers M, Perret M, Craig B M, Fang K S, Gustin M C. Mol Biol Cell. 2001;12:53–62. doi: 10.1091/mbc.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogakou E P, Pilch D R, Orr A H, Ivanova V S, Bonner W M. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 17.Rogakou E P, Nieves-Neira W, Boon C, Pommier Y, Bonner W M. J Biol Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 18.Rauchman M I, Nigam S K, Delpire E, Gullans S R. Am J Physiol. 1993;265:F416–F424. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- 19.Lee J C, Kassis S, Kumar S, Badger A, Adams J L. Pharmacol Ther. 1999;82:389–397. doi: 10.1016/s0163-7258(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 20.Pines J. Nat Cell Biol. 1999;1:E73–E79. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- 21.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 22.Furnari B, Rhind N, Russell P. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 23.Zhou B B, Chaturvedi P, Spring K, Scott S P, Johanson R A, Mishra R, Mattern M R, Winkler J D, Khanna K K. J Biol Chem. 2000;275:10342–10348. doi: 10.1074/jbc.275.14.10342. [DOI] [PubMed] [Google Scholar]
- 24.Taylor W R, Stark G R. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 25.Sarkaria J N, Busby E C, Tibbetts R S, Roos P, Taya Y, Karnitz L M, Abraham R T. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 26.Graves P R, Yu L, Schwarz J K, Gales J, Sausville E A, O'Connor P M, Piwnica-Worms H. J Biol Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- 27.Shackelford R E, Kaufmann W K, Paules R S. Environ Health Perspect. 1999;107(Suppl 1):5–24. doi: 10.1289/ehp.99107s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhl N M, Kunz J, Rensing L. Cell Prolif. 2000;33:147–166. doi: 10.1046/j.1365-2184.2000.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, Lee J D, Tobias P S, Ulevitch R J. J Biol Chem. 1993;268:25009–25014. [PubMed] [Google Scholar]
- 30.Freshney N W, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 31.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 32.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Chen C, Li Z, Guo W, Gegner J A, Lin S, Han J. J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 34.Allen M, Svensson L, Roach M, Hambor J, McNeish J, Gabel C A. J Exp Med. 2000;191:859–870. doi: 10.1084/jem.191.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brewster J L, de Valoir T, Dwyer N D, Winter E, Gustin M C. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- 36.Wojtaszek P A, Heasley L E, Berl T. J Clin Invest. 1998;102:1874–1881. doi: 10.1172/JCI4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida T, Sone M, Ogawa T, Nihei H, Ozasa H, Tsukada K, Horikawa S. Biochem Mol Biol Int. 1997;43:63–72. doi: 10.1080/15216549700203821. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Perez A, Burg M B. Physiol Rev. 1991;71:1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- 39.Kültz D, Garcia-Perez A, Ferraris J D, Burg M B. J Biol Chem. 1997;272:13165–13170. doi: 10.1074/jbc.272.20.13165. [DOI] [PubMed] [Google Scholar]
- 40.Denkert C, Warskulat U, Hensel F, Haussinger D. Arch Biochem Biophys. 1998;354:172–180. doi: 10.1006/abbi.1998.0661. [DOI] [PubMed] [Google Scholar]
- 41.Sheikh-Hamad D, Di Mari J, Suki W N, Safirstein R, Watts B A, III, Rouse D. J Biol Chem. 1998;273:1832–1837. doi: 10.1074/jbc.273.3.1832. [DOI] [PubMed] [Google Scholar]
- 42.Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H, Taya Y, Imai K. EMBO J. 2000;19:6517–6526. doi: 10.1093/emboj/19.23.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siderius M, Van Wuytswinkel O, Reijenga K A, Kelders M, Mager W H. Mol Microbiol. 2000;36:1381–1390. doi: 10.1046/j.1365-2958.2000.01955.x. [DOI] [PubMed] [Google Scholar]
- 44.Giaccia A J, Kastan M B. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]