Abstract
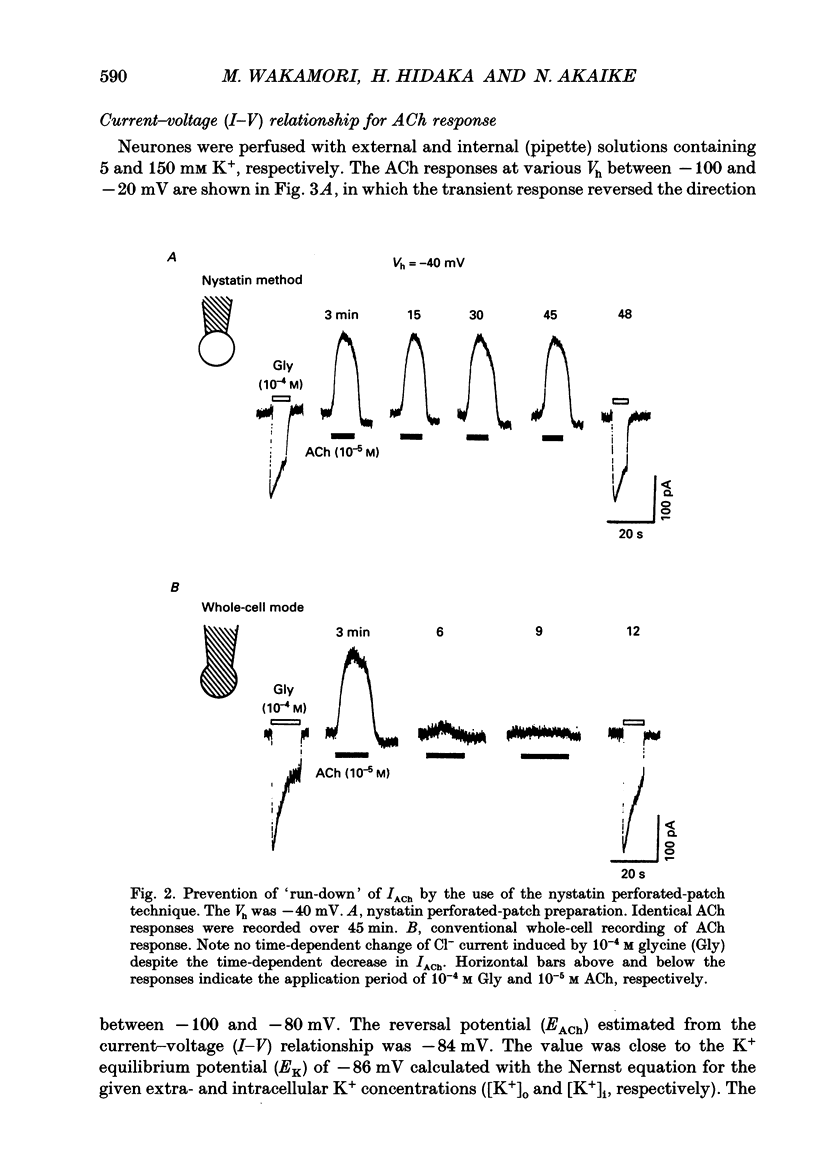
1. Intracellular mechanisms of the muscarinic acetylcholine (ACh) response were investigated in pyramidal neurones freshly dissociated from the rat hippocampal CA1 region. Current recordings were made in the whole-cell mode using the nystatin 'perforated'-patch technique, by which the muscarinic ACh response can be continuously recorded without so-called 'run-down' phenomenon. The amount of intracellular free Ca2+ ([Ca2+]i) was fluorometrically measured using fura-2. 2. In current clamp conditions, ACh induced a transient hyperpolarization accompanied by a decrease in membrane input resistance. 3. Under voltage clamp conditions at a holding potential (Vh) of -40 mV, ACh induced two types of muscarinic currents observed either alone or together: a transient outward current and a slowly activating sustained inward current. 4. The ACh-induced transient outward current reversed the direction at K+ equilibrium potential (EK), and the reversal potential (EACh) shifted 56.7 mV for a tenfold change of extracellular K+ concentration ([K+]o). 5. The ACh-induced transient outward current increased in a sigmoidal fashion with increase in ACh concentration, where the half-maximal concentration (EC50) and the Hill coefficient (n) were 8 x 10(-7) M and 1.9, respectively. Both muscarine and carbamylcholine mimicked the ACh response, but neither McN-A-343 (M1 agonist) nor oxotremorine (cardiac M2 agonist) induced any current. 6. Muscarinic antagonists reversibly blocked the ACh response in a concentration-dependent manner. The inhibitory potency was in the order of atropine > pirenzepine > AF-DX-116. 7. The ACh-induced transient outward current was never recorded when [Ca2+]i was chelated by the acetoxymethyl ester form of 1,2-bis(O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA AM). On the other hand, in Ca(2+)-free external solution containing 2 mM EGTA and 10 mM Mg2+, the ACh response was elicited by the first application and successive ACh applications did not induce any response. Fura-2 imaging showed that [Ca2+]i was increased when ACh was added to the external medium with or without Ca2+, though in Ca(2+)-free medium only the first application of ACh increased the [Ca2+]i. 8. The ACh response was not affected by pretreatment with pertussis toxin (PTX) but the inhibitory effect of ACh on the high-threshold Ca2+ channel was abolished completely. 9. Pretreatment with Li+ enhanced the amplitude of the transient outward current and the increase in [Ca2+]i induced by ACh. 10. The calmodulin antagonists W-7, chlorpromazine and trifluoperazine reversibly inhibited the ACh response in a concentration-dependent manner.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



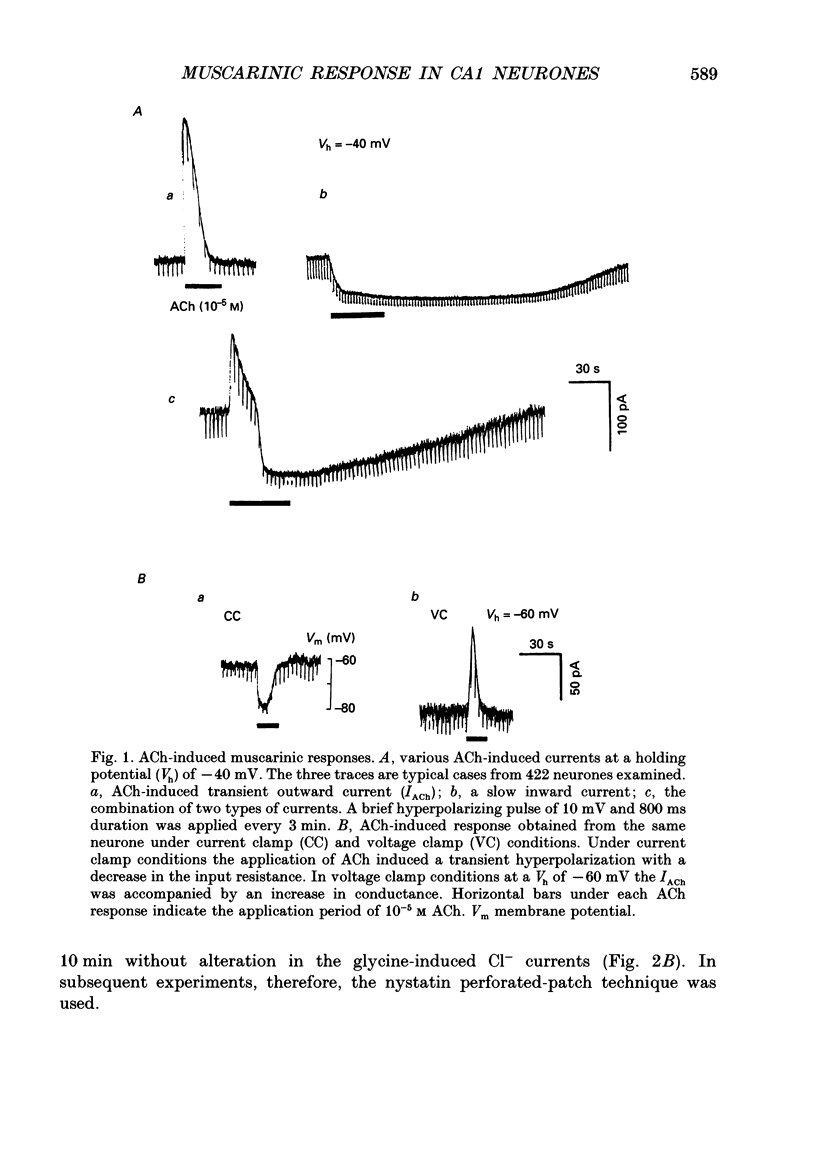
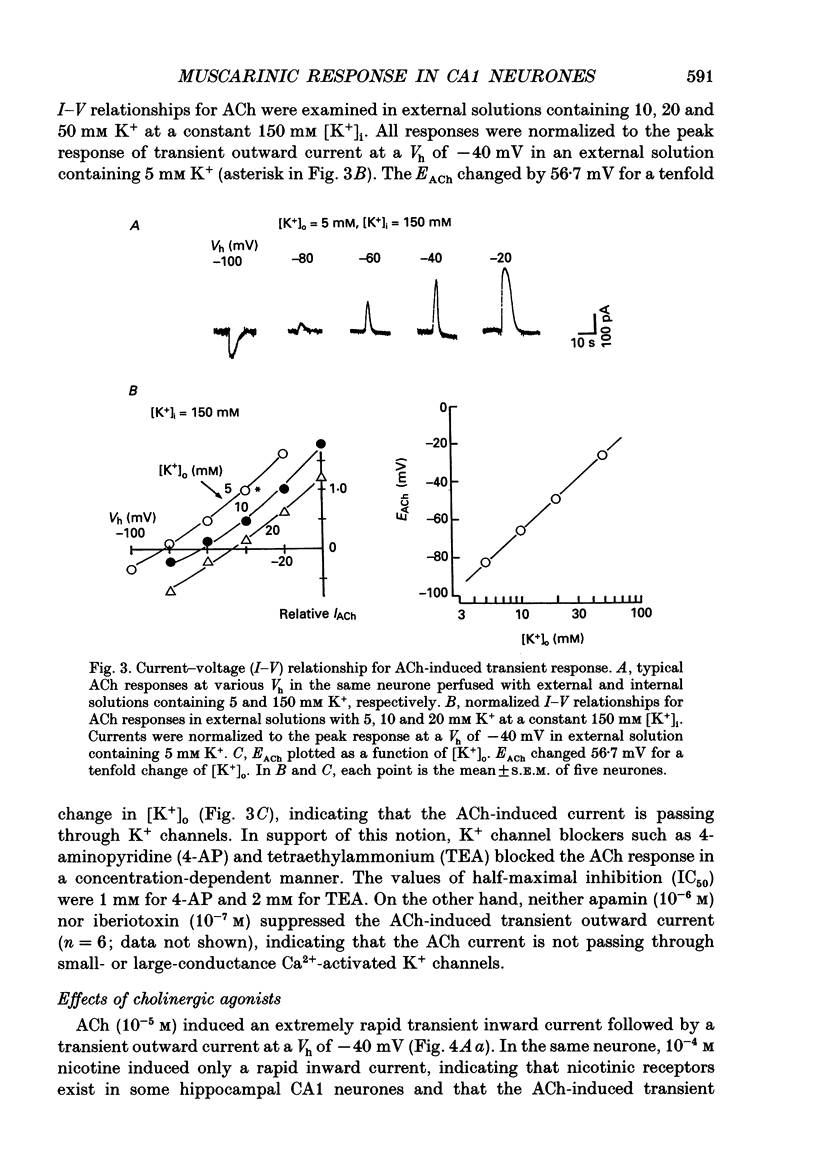





Selected References
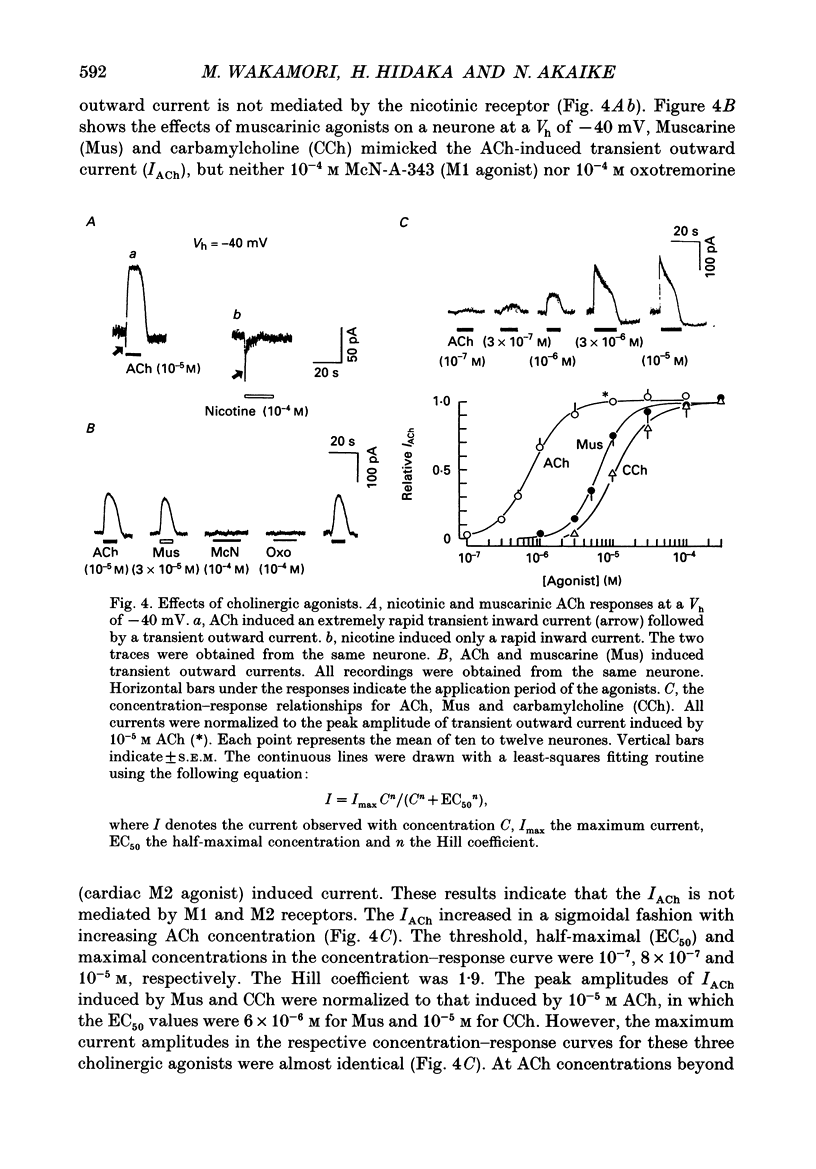
These references are in PubMed. This may not be the complete list of references from this article.
- Agopyan N., Agopyan I. Effects of protein kinase C activators and inhibitors on membrane properties, synaptic responses, and cholinergic actions in CA1 subfield of rat hippocampus in situ and in vitro. Synapse. 1991 Mar;7(3):193–206. doi: 10.1002/syn.890070304. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Avissar S., Schreiber G., Danon A., Belmaker R. H. Lithium inhibits adrenergic and cholinergic increases in GTP binding in rat cortex. Nature. 1988 Feb 4;331(6155):440–442. doi: 10.1038/331440a0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Krnjević K., Reinhardt W., Ropert N. Intracellular observations on the disinhibitory action of acetylcholine in the hippocampus. Neuroscience. 1981;6(12):2475–2484. doi: 10.1016/0306-4522(81)90093-2. [DOI] [PubMed] [Google Scholar]
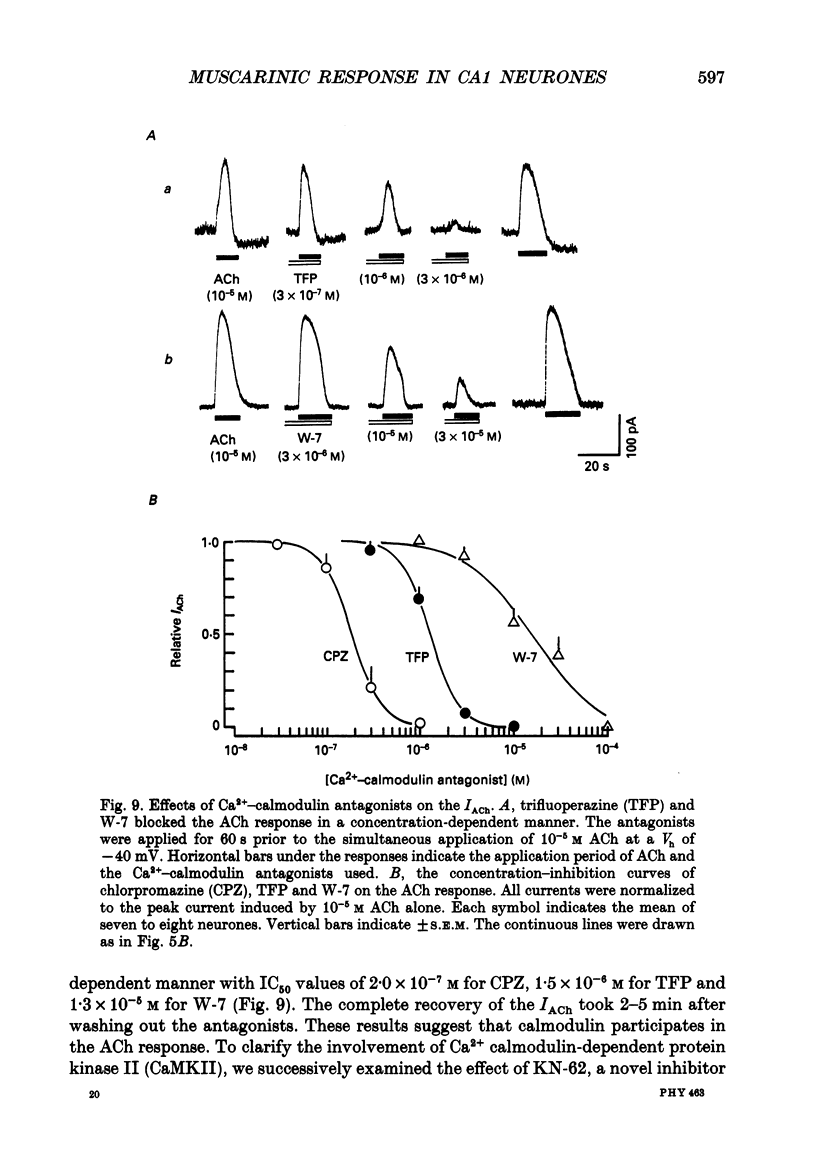
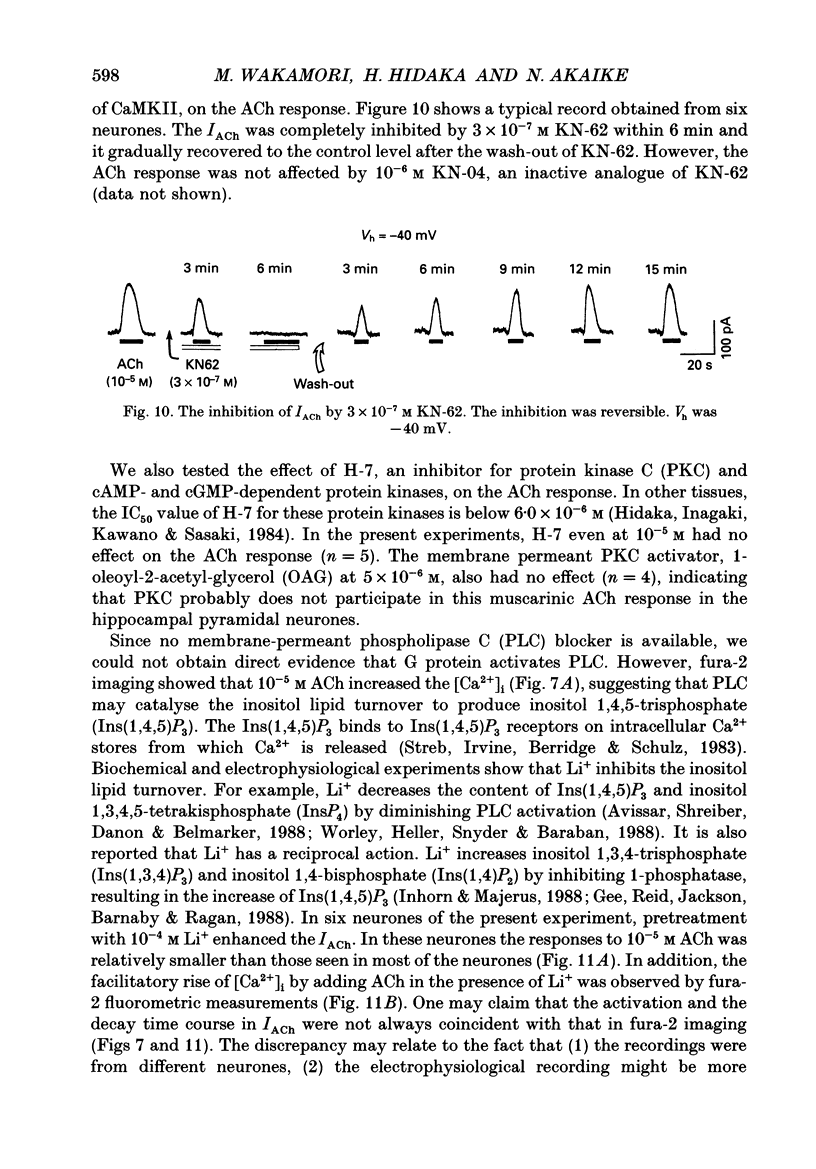
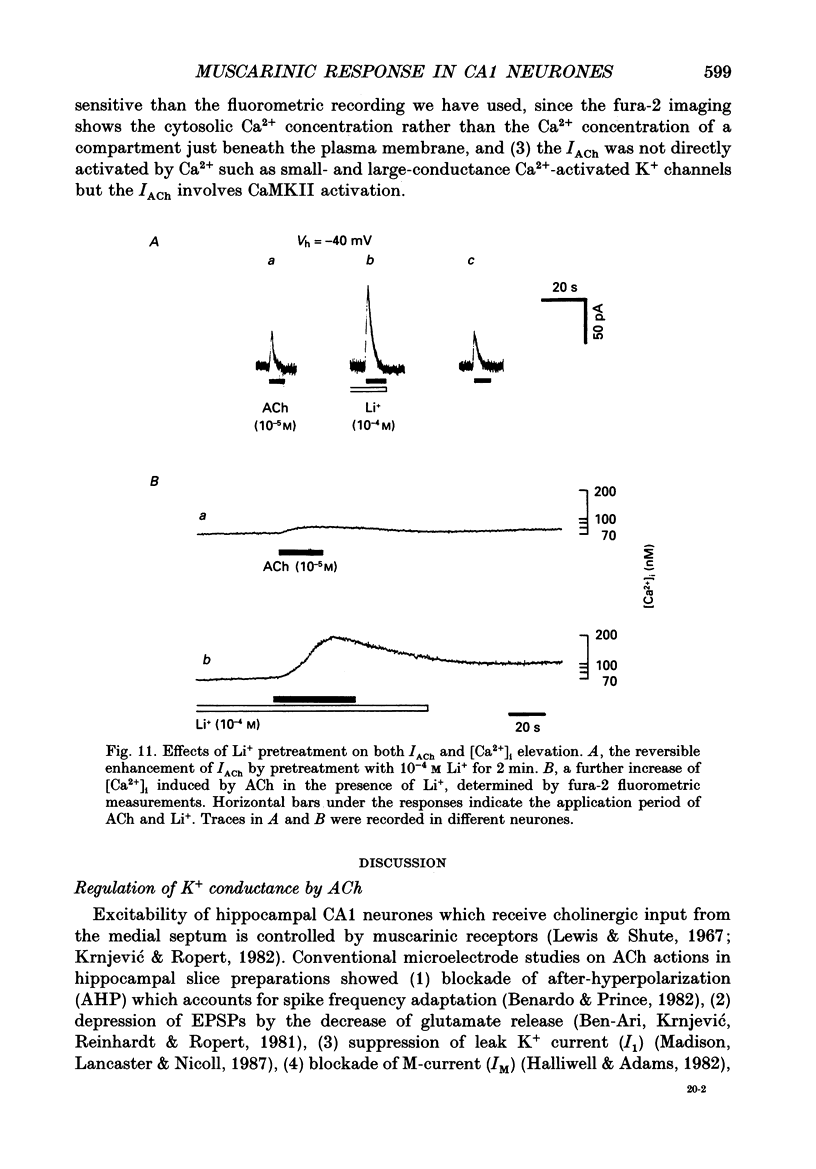
- Benardo L. S., Prince D. A. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):315–331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nairn A. C., Kuo J. F. Protein kinases 1988: a current perspective. FASEB J. 1988 Nov;2(14):2957–2969. doi: 10.1096/fasebj.2.14.2972578. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Buckley N. J., Jones S. V., Bonner T. I. Expression of a cloned muscarinic receptor in A9 L cells. Mol Pharmacol. 1987 Oct;32(4):450–455. [PubMed] [Google Scholar]
- Brown D. M-currents: an update. Trends Neurosci. 1988 Jul;11(7):294–299. doi: 10.1016/0166-2236(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Brann M. R. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988 Dec;8(12):4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. The pharmacology of cholinergic excitatory responses in hippocampal pyramidal cells. Brain Res. 1984 Jul 9;305(2):283–290. doi: 10.1016/0006-8993(84)90434-7. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Shinnick-Gallagher P. Muscarinic inhibitory transmission in mammalian sympathetic ganglia mediated by increased potassium conductance. Nature. 1984 Jan 19;307(5948):270–271. doi: 10.1038/307270a0. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Brann M. R., Buckley N. J., Ma A. L., Bonner T. I., Axelrod J. Stimulation of arachidonic acid release and inhibition of mitogenesis by cloned genes for muscarinic receptor subtypes stably expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8698–8702. doi: 10.1073/pnas.85.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., North R. A. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. 1986 Jan 30-Feb 5Nature. 319(6052):405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Higashida H., Kubo T., Maeda A., Akiba I., Bujo H., Mishina M., Numa S. Selective coupling with K+ currents of muscarinic acetylcholine receptor subtypes in NG108-15 cells. Nature. 1988 Sep 22;335(6188):355–358. doi: 10.1038/335355a0. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kubo T., Akiba I., Maeda A., Mishina M., Numa S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987 Jun 18;327(6123):623–625. doi: 10.1038/327623a0. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Reid G. G., Jackson R. G., Barnaby R. J., Ragan C. I. Purification and properties of inositol-1,4-bisphosphatase from bovine brain. Biochem J. 1988 Aug 1;253(3):777–782. doi: 10.1042/bj2530777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Haga T., Ichiyama A., Katada T., Kurose H., Ui M. Functional reconstitution of purified muscarinic receptors and inhibitory guanine nucleotide regulatory protein. Nature. 1985 Aug 22;316(6030):731–733. doi: 10.1038/316731a0. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harata N., Tateishi N., Akaike N. Acetylcholine receptors in dissociated nucleus basalis of Meynert neurons of the rat. Neurosci Lett. 1991 Sep 16;130(2):153–156. doi: 10.1016/0304-3940(91)90385-7. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn R. C., Majerus P. W. Properties of inositol polyphosphate 1-phosphatase. J Biol Chem. 1988 Oct 5;263(28):14559–14565. [PubMed] [Google Scholar]
- Ito C., Wakamori M., Akaike N. Dual effect of glycine on isolated rat suprachiasmatic neurons. Am J Physiol. 1991 Feb;260(2 Pt 1):C213–C218. doi: 10.1152/ajpcell.1991.260.2.C213. [DOI] [PubMed] [Google Scholar]
- Kanterman R. Y., Ma A. L., Briley E. M., Axelrod J., Felder C. C. Muscarinic receptors mediate the release of arachidonic acid from spinal cord and hippocampal neurons in primary culture. Neurosci Lett. 1990 Oct 16;118(2):235–237. doi: 10.1016/0304-3940(90)90635-m. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Ropert N. Electrophysiological and pharmacological characteristics of facilitation of hippocampal population spikes by stimulation of the medial septum. Neuroscience. 1982;7(9):2165–2183. doi: 10.1016/0306-4522(82)90128-2. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system, and the subfornical organ and supra-optic crest. Brain. 1967 Sep;90(3):521–540. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Lancaster B., Nicoll R. A. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987 Mar;7(3):733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. Activation and desensitization mechanisms of muscarinic current response in single pancreatic acinar cells of rats. J Physiol. 1989 Oct;417:343–359. doi: 10.1113/jphysiol.1989.sp017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. 1986 Jan 30-Feb 5Nature. 319(6052):402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- Murase K., Randic M., Shirasaki T., Nakagawa T., Akaike N. Serotonin suppresses N-methyl-D-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Res. 1990 Aug 13;525(1):84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A., Fukuda K., Kubo T., Numa S. Intracellular calcium release mediated by two muscarinic receptor subtypes. FEBS Lett. 1988 Nov 21;240(1-2):88–94. doi: 10.1016/0014-5793(88)80345-4. [DOI] [PubMed] [Google Scholar]
- Onozuka M., Furuichi H., Imai S., Fukami Y. Evidence that Ca2+/calmodulin-dependent protein phosphorylation is involved in the opening process of potassium channels in identified snail neurons. Neurosci Lett. 1991 Mar 11;124(1):35–38. doi: 10.1016/0304-3940(91)90816-c. [DOI] [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Ramachandran J., Capon D. J. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988 Aug 4;334(6181):434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- Segal M. Multiple action of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res. 1982 Aug 19;246(1):77–87. doi: 10.1016/0006-8993(82)90144-5. [DOI] [PubMed] [Google Scholar]
- Spencer D. G., Jr, Horváth E., Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res. 1986 Aug 13;380(1):59–68. doi: 10.1016/0006-8993(86)91429-0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990 Mar 15;265(8):4315–4320. [PubMed] [Google Scholar]
- Toselli M., Lang J., Costa T., Lux H. D. Direct modulation of voltage-dependent calcium channels by muscarinic activation of a pertussis toxin-sensitive G-protein in hippocampal neurons. Pflugers Arch. 1989 Dec;415(3):255–261. doi: 10.1007/BF00370874. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Heller W. A., Snyder S. H., Baraban J. M. Lithium blocks a phosphoinositide-mediated cholinergic response in hippocampal slices. Science. 1988 Mar 18;239(4846):1428–1429. doi: 10.1126/science.2831626. [DOI] [PubMed] [Google Scholar]


