Abstract
The stimulatory effects of growth hormone-releasing hormone (GHRH) and the antiproliferative action of GHRH antagonists have been demonstrated in various cancers, but the receptors that mediate these responses are not clearly identified. Recently, we reported that human cancer cell lines express splice variants (SVs) of the receptors for GHRH. SV1 exhibits the greatest similarity to the pituitary GHRH receptor and is most likely to be functional. To ascertain whether SV1 mediates mitogenic effects on nonpituitary tissues, we expressed SV1 in 3T3 mouse fibroblasts and studied the properties of the transfected cells. Radioligand binding assays with 125I-labeled GHRH antagonist JV-1–42 detected high affinity (Kd = 0.58 ± 0.17 nM) binding sites for GHRH with a maximal binding capacity (Bmax) of 103 ± 17.4 fmol/mg of membrane protein in 3T3 cells transfected with pcDNA3-SV1, whereas the control cells transfected with the empty vector did not show any GHRH binding. Cell proliferation studies showed that cells expressing SV1 are much more sensitive to GHRH analogs than the pcDNA3 controls. Thus, the expression of SV1 augments the stimulatory responses to GHRH(1–29)NH2 or GHRH agonist JI-38 and inhibitory responses to GHRH antagonist JV-1–38 as compared with pcDNA3 controls. The stimulation of SV1-expressing cells by GHRH or JI-38 is followed by an increase in cAMP production, but no GH release occurs. Vasoactive intestinal peptide had no effect, and its antagonist JV-1–53 did not inhibit the proliferation of SV1-expressing cells stimulated by GHRH. Our results suggest that SV1 could mediate responses of nonpituitary cells and various tumors to GHRH and GHRH antagonists. The presence of SV1 in several human cancer cell lines provides a rationale for antitumor therapy based on the blockade of this receptor by specific GHRH antagonists.
Growth hormone-releasing hormone (GHRH) is secreted by the hypothalamus and upon binding to specific GHRH receptors in the pituitary stimulates the synthesis and the release of GH. GH in turn induces the production in the liver of insulin-like growth factor (IGF-I), which is a known mitogen for various cell types (1). GHRH antagonists strongly suppress the growth of various experimental cancers such as osteosarcomas (ref. 2 and R. Braczkowski, A.V.S., A. Plonowski, J.L.V., K. Groot, M. Krupa, and P. Armatis, unpublished observations), renal cell carcinomas (3, 4), prostatic cancers (5, 6), small cell lung carcinomas (SCLC) and non-SCLC (7, 8), malignant glioblastomas (9), and pancreatic (10), colorectal (11), breast (12–14), and ovarian tumors (15). Treatment with GHRH antagonists can produce a marked reduction in the serum levels of IGF-I in the tumor-bearing animals, consistent with the notion that the antitumor action of the antagonistic analogs of GHRH is exerted in part through an indirect mechanism involving the inhibition of GHRH/GH/IGF-I axis (16, 17). However, accumulating evidence suggests that antitumor effects are also mediated through a direct mechanism without the involvement of pituitary GH and hepatic IGF-I. This concept is upheld by the observations that various experimental tumors, including SCLCs and ovarian and breast cancer lines, are inhibited by GHRH antagonists while being IGF-I independent, and that the tumor growth inhibition after administration of GHRH antagonists is not always associated with decreased levels of IGF-I in the serum of the tumor-bearing animals (7, 13, 15). The direct action of GHRH analogs on diverse cancers is also supported by studies that show GHRH antagonists inhibit the proliferation of various cancer cell lines, such as H-69 SCLCs, OV-1063 ovarian carcinoma, MNNG/HOS and SK-ES-1 osteosarcoma, and MXT mouse mammary cancers cultured in vitro (7, 14, 15, 18). Furthermore, ectopic or autocrine/paracrine production of GHRH has been reported in clinical and experimental studies involving pancreatic, lung, ovarian, and prostate cancers, adding further evidence to the notion that GHRH may play a direct role in carcinogenesis (19–23).
The nature of tumoral receptors that mediate the direct effects of the GHRH and GHRH antagonists on cell proliferation has not been clarified because the receptors for GHRH in the pituitary form could not be detected in any of the cancer models, although distinct responses to GHRH antagonists by a direct mechanism were recorded (16, 17). However, it has been reported recently that various cancer cell lines, including LNCaP, PC-3, ALVA-41, DU-145, and MDA-PCa-2b prostatic, MiaPaCa-2 pancreatic, CAKI-1 renal, H-69 SCLC, MDA-MB-435 breast cancers, OV-1063 ovarian carcinoma, and MNNG/HOS and SK-ES-1 osteosarcoma (4, 13, 15, 18, 23–25) express splice variants (SVs) of GHRH receptor, encoding alternate forms of GHRH receptor, although their functionality remains unknown. Primary tumor specimens from patients with prostate and lung cancer (G.H., A.V.S., and R.B., unpublished results) also express these SVs. Among these truncated forms of GHRH receptor, SV1 exhibits the greatest similarity to the pituitary GHRH receptor and is predominantly detected in most of the tumor samples tested. SV1 and the pituitary GHRH receptor differ only in the first 3 exons, encoding a part of the extracellular domain of the receptor that in SV1 has been replaced by a fragment of intron 3, which has a new putative in-frame start codon (25).
The aim of the present study was to investigate whether SV1 can mediate the effects of GHRH analogs on cell proliferation in nonpituitary tissues. We expressed SV1 in NIH 3T3 mouse fibroblasts and evaluated the binding of GHRH analogs, mitogenic actions of GHRH(1–29)NH2 and GHRH agonist JI-38 (26), and the antiproliferative responses to the GHRH antagonist JV-1–38 (27) as well as the effects of vasoactive intestinal peptide (VIP) and VIP antagonist JV-1–53.
Materials and Methods
Peptides.
hGHRH(1–29)NH2, GHRH antagonists JV-1–38 and JV-1–42, GHRH agonist JI-38, and VIP antagonist JV-1–53 were synthesized in our laboratory by solid phase method and purified as described (4, 26, 27). VIP was obtained from California Peptide Research (Napa, CA).
RNA Extraction and Reverse Transcription–PCR.
Total RNA was extracted from cells by using the Tri-Reagent (Sigma) according to manufacturer's instructions. RNA was reverse transcribed into cDNA as described previously (25). The PCR amplification of cDNA for the evaluation of the expression of SV1 was performed as follows. One microliter of cDNA was amplified in a 25-μl mixture containing 1 × PCR buffer, 2 mM MgCl2/200 μM of each dNTP/0.5 μM of each primer/2.5 units/100 μl AmpliTaq DNA polymerase. The primers used were 5′-GCACCTTTGAAGCAGAGAGG-3′ (sense) and 5′-CACGTGCAGTGAAGAGCACGG-3′ (antisense) producing a PCR product of 720 bp (25). PCR consisted of 1 cycle at 95°C for 3 min, followed by 25 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min and a final extension at 72°C for 7 min by using a Robocycler system (Stratagene). Negative controls included omission of reverse transcriptase and substitution of template with water. The PCR products were electrophoresed on 1.5% agarose gel and stained with ethidium bromide.
Construction of Plasmids.
SV1 was amplified from LNCaP prostate cancer cell line by reverse transcription–PCR as described above by using the primers 5′-CCTACTGCCCTTAGGATGCTGG-3′ and 5′-CCCTTGCTCCTCCAGAGCATGG-3′. The resulting 1,371-bp PCR product was subcloned into the vector pCR-2.1 (Invitrogen) by using the TOPO-TA cloning kit (Invitrogen) according to manufacturer's instructions. Subsequently, SV1 was subcloned into the KpnI–NotI sites of the vector pcDNA-3 (Invitrogen) by using standard procedures (28).
Cell Culture.
NIH 3T3 mouse fibroblasts were obtained from American Type Culture Collection and were routinely grown as a monolayer in DMEM supplemented with 10% FBS/100 units/ml penicillin/100 mg/ml streptomycin/100 units/ml amphotericin B at 37°C in a humidified 95% air/5% carbon dioxide atmosphere. Cells were passaged weekly and monitored routinely for mycoplasma contamination by using a detection kit (Boehringer Mannheim). All tissue culture reagents were obtained from GIBCO unless otherwise stated. The generation of cells expressing SV1 was performed by transfecting pcDNA3-SV1 construct or empty vector (controls), by using the FuGENE 6 Transfection Reagent (Roche Diagnostics) according to manufacturer's instructions. The selection of the resistant clones was performed after exposure of the transfected cells to G-418 (GIBCO) at 500 μg/ml for 2 weeks. The determination of the rate of cell proliferation was performed by the crystal violet assay after exposure of cells to the peptide analogs for 72 h at appropriate concentrations (29). The results were calculated as percent T/C, where T = optical density (OD600 nm) of treated cultures and C = OD600 nm of control cultures × 100. Measured absorbance is proportionate to cell number. During the cell proliferation experiments, the cells were cultured in QBSF-51 serum-free media (Sigma).
Measurement of cAMP Levels.
Cells were seeded in 12-well plates (2 × 104 cells per well) with DMEM containing 10% FBS and cultured until reaching 70–80% confluency. Subsequently, cells were incubated at 37°C for 30 min with DMEM containing 1 mg/ml bacitracin/0.5% (wt/vol) BSA/0.05 mM phenylmethylsulfonyl fluoride/0.4 mM 3-isobutyl-I-methyl-xanthine/1 μM GHRH(1–29)NH2 or GHRH agonist JI-38. Medium was then removed and acetylated with triethylamine/acetic anhydride (2:1; 25 μl per 500-μl sample). 2′-O-monosuccinyl-cAMP tyrosyl methyl ester (Sigma) was used for the iodination. The antiserum for cAMP was obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (CV-27). cAMP from Sigma was used as the standard.
Measurement of GH Levels.
Cells were washed twice with prewarmed DMEM without serum and then the same medium containing hGHRH(1–29)NH2 or GHRH antagonist JV-1–38 at 1 μM. The medium was collected at 60 min and 120 min after the addition of the ligands, and the samples were frozen at −20°C until assayed for mGH. Measurement of mGH levels was performed by RIA as described (15).
Ligand-Binding Assay.
The radioiodinated derivative of GHRH antagonist JV-1–42 was prepared by the chloramine T method as described (4). Preparation of the membrane fractions from 250–330 million control or pcDNA-3-SV1 transfected cells was carried out as reported (4). Receptor binding of GHRH was performed by using in vitro ligand competition assays based on the binding of [125I]JV-1–42 to membrane fractions of the cells as described (4). The characteristics of the specific ligand binding were determined with the LIGAND-PC curve-fitting software and by Scatchard analysis.
Statistical Analysis.
The data are expressed as the mean ± SEM. Statistical evaluation of the data were performed by the Student's t test (two-tailed). Differences were considered statistically significant when P < 0.05. P values shown are against the control group unless otherwise stated.
Results
SV1 Expression and Ligand Binding.
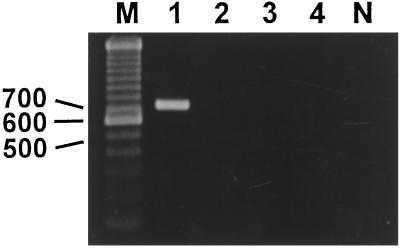
Reverse transcription–PCR analysis confirmed that 3T3 cells transfected with pcDNA-3-SV1 express the mRNA for SV1 (Fig. 1). Using complete displacement analyses with 125I-labeled GHRH antagonist JV-1–42 as radioligand, we could not detect any GHRH binding in control cells. In contrast, using the same GHRH radioligand we were able to detect high affinity (Kd = 0.58 ± 0.17 nM) binding sites for GHRH, with a maximal binding capacity of 103 ± 17.4 fmol/mg membrane protein in membrane fraction of 3T3 cells transfected with pcDNA-3-SV1.
Figure 1.
Reverse transcription–PCR analysis of expression of mRNA for GHRH receptor splice variant I. A product of the expected size of 720 bp was found in NIH 3T3 cells stably transfected with pcDNA3-SVI (lane 1). No expression of SV1 of GHRH receptor mRNA was detected in 3T3 cells stably transfected with pcDNA3 (empty vector) (lane 2). Lanes containing PCR products obtained without reverse transcription (lanes 3 and 4, 3T3-SV1 transfected and control cells, respectively) or using water as template (lane N) were also negative. Lane M, molecular weight marker.
Effect of GHRH, GHRH Agonist JI-38, and GHRH Antagonist JV-1–38 on the Proliferation of 3T3 Cells Expressing SV1.
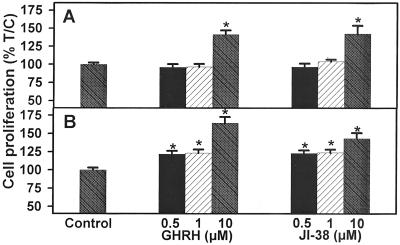
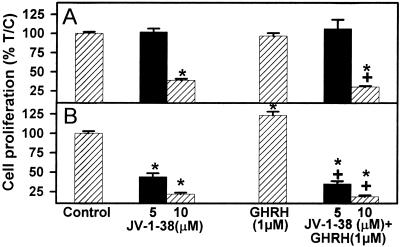
3T3 cells expressing SV1 and controls transfected with the empty vector were exposed to various concentrations of GHRH(1–29)NH2, GHRH agonist JI-38, and GHRH antagonist JV-1–38, and the rate of cell proliferation was measured by the crystal violet assay. As shown in Figs. 2 and 3 and Table 1, the expression of SV1 increased the sensitivity of 3T3 cells to GHRH analogs. Both GHRH(1–29)NH2 and GHRH agonist JI-38, at a concentration of 0.5–1 μM, stimulated the proliferation of 3T3 cells expressing SV1 by about 20–23% (P < 0.001) without affecting the growth of control cells transfected with the empty vector (Fig. 2, Table 1). The proliferation of the SV1-expressing cells was stimulated by 40–65% (P < 0.001) in the presence of 10 μM GHRH(1–29)NH2 or JI-38. The growth of the control 3T3 cells was also increased at this concentration of GHRH or its agonist (Fig. 2, Table 1). JV-1–38, an antagonistic analog of GHRH, at a concentration of 5 μM inhibited the proliferation of the SV1-expressing cells by 57% (P < 0.001) but did not affect the growth of the control cells (Table 1, Fig. 3). However, the proliferation of both the SV1-expressing cells and the controls was significantly inhibited by 79% and 62% (P < 0.001), respectively, by 10 μM concentration of this antagonist (Table 1, Fig. 3).
Figure 2.
Effect of GHRH(1–29)NH2 and GHRH agonist JI-38 on the proliferation of 3T3 cells stably transfected with (A) pcDNA3 (control cells) or (B) pcDNA3-SV1. Vertical bars represent SEM. *, P < 0.001 vs. control. Relative cell number in treated and control plates was determined by crystal violet staining and expressed as percent T/C values, where T = absorbance of treated cultures and C = absorbance of control cultures. Measured absorbance is proportionate to cell number.
Figure 3.
Effect of GHRH antagonist JV-1–38, alone or in combination with GHRH(1–29)NH2 at 1 μM concentration, on the proliferation of 3T3 cells stably transfected with (A) pcDNA3 (control cells) or (B) pcDNA3-SV1. Vertical bars represent SEM. *, P < 0.001 vs. control; +, P < 0.001 vs. GHRH(1–29)NH2.
Table 1.
Effect of GHRH, VIP, and their analogs on the proliferation of NIH 3T3 mouse fibroblasts transfected with pcDNA3-SV1 or pcDNA3 (control cells)
| 3T3 cells pcDNA3 proliferation % | P value vs. control | 3T3 cells pcDNA3-SV1 proliferation % | P value vs. control | P value SV1 3T3 cells vs. control cells | |
|---|---|---|---|---|---|
| Control | 100 ± 2.19 | 100 ± 2.84 | ns | ||
| GHRH(1–29) (0.5 μM) | 95.89 ± 4.41 | ns | 121.03 ± 4.35 | <0.001 | <0.001 |
| GHRH(1–29) (1 μM) | 96.48 ± 4.04 | ns | 123.39 ± 4.88 | <0.001 | <0.001 |
| GHRH(1–29) (10 μM) | 141.27 ± 5.83 | <0.001 | 164.74 ± 8.05 | <0.001 | <0.05 |
| GHRH agonist JI-38 (0.5 μM) | 96.35 ± 4.80 | ns | 122.00 ± 4.15 | <0.001 | <0.001 |
| JI-38 (1 μM) | 103.30 ± 3.06 | ns | 123.64 ± 4.42 | <0.001 | <0.001 |
| JI-38 (10 μM) | 142.53 ± 11.15 | <0.001 | 143.32 ± 7.60 | <0.001 | ns |
| GHRH antagonist JV-1-38 (5 μM) | 101.76 ± 4.50 | ns | 43.86 ± 4.80 | <0.001 | <0.001 |
| JV-1-38 (10 μM) | 38.02 ± 2.18 | <0.001 | 21.64 ± 2.09 | <0.001 | <0.001 |
| GHRH(1–29) (1 μM) + JV-1-38 (5 μM) | 106.13 ± 12.00 | ns | 35.16 ± 3.55 | <0.001 | <0.001 |
| GHRH (1–29) (1 μM) + JV-1-38 (10 μM) | 30.33 ± 1.41 | <0.001 | 18.45 ± 2.05 | <0.001 | <0.001 |
| VIP (1 μM) | 102.08 ± 3.34 | ns | 104.30 ± 2.15 | ns | ns |
| VIP antagonist JV-1-53 (3 μM) | 109.90 ± 5.05 | ns | 102.67 ± 2.97 | ns | ns |
| JV-1-53 (5 μM) | 112.79 ± 5.30 | <0.02 | 108.79 ± 2.37 | <0.05 | ns |
| JV-1-53 (10 μM) | 91.36 ± 9.36 | ns | 66.17 ± 4.62 | <0.001 | <0.02 |
| GHRH(1–29) (1 μM) + JV-1-53 (3 μM) | 106.11 ± 2.91 | ns | 121.71 ± 4.07 | <0.001 | <0.01 |
| GHRH(1–29) (1 μM) + JV-1-53 (5 μM) | 111.95 ± 3.50 | <0.01 | 128.67 ± 3.85 | <0.001 | <0.01 |
| GHRH(1–29) (1 μM) + JV-1-53 (10 μM) | 82.58 ± 11.01 | <0.05 | 88.13 ± 5.89 | <0.05 | ns |
Data are expressed as mean (%) ± SEM. Statistical analyses were performed by using the Student two-tailed t test. ns, not significant.
The inhibitory effect of 5 and 10 μM GHRH antagonist JV-1–38 on growth of 3T3 cells expressing SV1 was not reversed in the presence of 1 μM GHRH(1–29)NH2, the resulting suppression being 65% (P < 0.001) and 81% (P < 0.001) respectively (Table 1, Fig. 3). The growth of control cells in the presence of 1 μM GHRH(1–29)NH2 was only inhibited by the higher concentration (10 μM) of JV-1–38 (Table 1, Fig. 3).
Effect of VIP and VIP Antagonist JV-1–53 on the Proliferation of 3T3 Cells Expressing SV1.
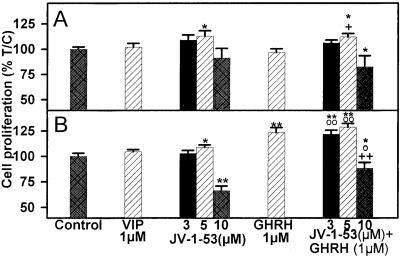
As shown in Fig. 4 and Table 1, VIP at 1 μM concentration had no effect on the proliferation of 3T3 cells, transfected or not with SV1. VIP antagonist JV-1–53 at 5 μM did not inhibit the growth of either the SV1-transfected cells or the control cells, but at a concentration of 10 μM, it suppressed only the proliferation of the cells expressing SV1. We also tested whether JV-1–53 could suppress the mitogenic effect of GHRH(1–29)NH2 on the SV1-expressing cells. Exposure of 3T3 cells expressing SV1 to 1 μM GHRH(1–29)NH2 in the presence of 3–5 μM JV-1–53 failed to inhibit the GHRH-induced stimulation of cell proliferation, which was increased by 21% (P < 0.001) and 28% (P < 0.001), respectively, vs. controls (Fig. 4, Table 1). However, addition of 10 μM JV-1–53 to the culture medium could inhibit cell proliferation by 12% (P < 0.05) in the presence of 1 μM GHRH(1–29)NH2 (Fig. 4, Table 1). When a similar experiment was performed with the control cells, 1 μM GHRH(1–29)NH2 did not alter the effect of JV-1–53 on cell proliferation at concentrations tested (Fig. 4, Table 1).
Figure 4.
Effect of VIP and VIP antagonist JV-1–53, alone or in combination with GHRH(1–29)NH2 at 1 μM, on the proliferation of 3T3 cells stably transfected with (A) pcDNA3 (control cells) or (B) pcDNA3-SV1. Vertical bars represent SEM. *, P < 0.05 and **, P < 0.001 vs. control; ○, P < 0.05 and ○○, P < 0.001 vs. JV-1–53; +, P < 0.05 and ++, P < 0.001 vs. GHRH(1–29)NH2.
Effect of GHRH(1–29)NH2 and GHRH Agonist JI-38 on cAMP Production and GH Release in 3T3 Cells Expressing SV1.
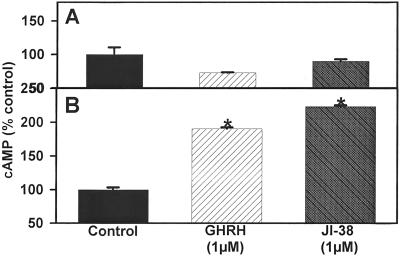
Because GHRH receptor is coupled to adenylyl cyclase, we evaluated the effect of GHRH(1–29)NH2 or GHRH agonist JI-38 at 1 μM concentration on the levels of cAMP in the media from 3T3 cells expressing SV1 and control cells transfected with the empty vector. As shown in Fig. 5, both GHRH(1–29)NH2 and JI-38 stimulated significantly (P < 0.001) the production of cAMP in the SV1-expressing cells by about 90% and 120% as compared with the controls. The cAMP levels in the cells transfected with the empty vector did not change after exposure to GHRH(1–29)NH2 or JI-38.
Figure 5.
Effect of GHRH(1–29)NH2 and GHRH agonist JI-38 at 1 μM, on cAMP production of 3T3 cells stably transfected with (A) pcDNA3 (control cells) or (B) pcDNA3-SV1. Vertical bars represent SEM. *, P < 0.001.
We also investigated whether SV1 could activate GH secretion. The basal levels of mGH in the control cells were 410 ± 61.72 pg/ml and in the SV1 transfected cells 398 ± 24.09 pg/ml. SV1-expressing and control cells were exposed to GHRH(1–29)NH2 or GHRH antagonist JV-1–38 at 1 μM for 60 min and 120 min, and the levels of GH in the media were measured. Neither type of cells showed GH secretory response to GHRH stimulation or inhibition (data not shown).
Discussion
The splice variant SV1 for GHRH receptor exhibits a higher similarity to the full-length pituitary GHRH receptor than other truncated forms and is the most frequently expressed in a broad spectrum of cancer cell lines. In the present study, we sought to examine whether a stable expression of SV1 on cells that normally do not express it could activate their responsiveness to GHRH analogs. Thus, we evaluated whether the expression of this splice variant in NIH 3T3 mouse fibroblasts alters their responses to GHRH analogs and their growth characteristics. Our results show that the SV1 isoform of GHRH receptor gene can be translated into high affinity GHRH binding sites, thus providing evidence that the mRNA of SV1 detected previously on various cancer lines encodes a specific GHRH receptor determined by radioligand assays (4, 25). A role for SV1 in mediating cell proliferation is supported by the finding that cells expressing SV1 are more sensitive than the controls to the mitogenic signal transduced by GHRH and its agonistic analog JI-38, whereas GHRH antagonist JV-1–38 strongly inhibits their growth. The antiproliferative effect of 5 μM and 10 μM concentrations of JV-1–38 in SV1-transfected cells was not nullified by 1 μM GHRH(1–29)NH2, and this GHRH antagonist produced essentially the same inhibition of cell growth in the presence or absence of GHRH(1–29)NH2. This is in accordance with the fact that JV-1–38 is a potent GHRH antagonist whose binding affinity to the tumoral and pituitary GHRH receptors is 20–40-fold higher than that of the native GHRH (4, 27). On the other hand, GHRH(1–29)NH2 stimulated cell proliferation in the presence of the specific VIP antagonist JV-1–53, used as a negative control in the present work. In a previous study on GHRH antagonists, we obtained similar results by using crystal violet assay and by measurement of thymidine incorporation (30). Thus, the findings of the present study based on the crystal violet assay should reflect differences in the rate of cell proliferation and not the rate of cell death. Apart from the augmentation of cell growth, the stimulation of SV1-transfected cells by GHRH or its agonist JI-38 also induces the production of cAMP, but not the release of GH. It seems that the expression of SV1 in nonpituitary cells such as 3T3 fibroblasts is sufficient for activating the GHRH receptor-specific cAMP-coupled signal transduction pathways by GHRH (31). Because it has been shown previously that cAMP decreases the proliferation of 3T3 cells (32), the rise of cAMP seen in our study is not likely to have contributed to the stimulatory effect of GHRH on cell growth. The absence of a GH release response is most likely because of the fact that 3T3 fibroblasts are morphologically completely different from pituitary somatotrophs, which are able to acutely release GH upon various stimuli. The amounts of GH released by either type of 3T3 cells are estimated to be only 0.003–0.01% of the pituitary GH release.
In SV1, a part of the extracellular protein, corresponding to the portion of the receptor encoded by the first 3 exons of the gene, has been replaced by a sequence encoded by the 3rd intron. It seems that the specificity for recognizing GHRH remains unaffected in SV1, not only because its binding of GHRH analogs is highly specific but also because cells expressing SV1 are much more sensitive to GHRH than to VIP, a mitogenic peptide homologous in structure to GHRH. This is in agreement with previous findings demonstrating that although the extracellular domain of the receptor is necessary for ligand binding, it is the transmembrane portion that determines the ligand specificity (33).
Several possible mechanisms may be involved in the ligand-independent inhibitory proliferative effect observed in the SV1-expressing cells. First, and less probable, is that the 3T3 cells express GHRH-like ligands whose activity is not manifested in these cells because they fail to express the appropriate receptors. The expression of SV1 could provide a receptor for these ligands. However, it is more likely that expression of SV1 represents a form of GHRH receptor that retains the ability to specifically bind the GHRH analogs and subsequently to potentiate mitogenic signals. This suggestion is supported by the observation that SV1-expressing cells are much more sensitive to the inhibitory effect of GHRH antagonist JV-1–38 than to the stimulatory effects of GHRH and its agonist JI-38, as reflected in the effects on the rate of cell proliferation. In addition, GHRH antagonist JV-1–38, when assayed by rat pituitary superfusion experiments for its inhibitory effect on the GHRH-induced GH release, does not exhibit considerable intrinsic GHRH agonistic activity (27). These possibilities remain to be further tested by comparing the baseline activity of SV1 versus the full-length GHRH receptor in a cell system similar to that used in the present study and/or by investigating how the direct inhibition of this receptor, probably by antisense RNA-based strategy, affects cell proliferation. An additional explanation for our findings is that expression of SV1 stimulates the expression of the full-length GHRH receptor in 3T3 cells, which in turn mediates the effects of the GHRH analogs in the SV1-expressing cells. Although this possibility cannot be ruled out by the present experiments, it is unlikely because in various cell lines in which we demonstrated expression of the endogenous SV1, we failed to detect the full-length GHRH receptor (4, 13, 15, 18, 23–25).
The present study shows that SV1, the main isoform of GHRH receptor, expressed in human tumors can mediate mitogenic effects in nonpituitary cells such as the 3T3 fibroblasts upon its transfection. These findings directly support the association between the ectopic production of GHRH with carcinogenesis in nonpituitary tissues (19–23) and suggest the presence of an autocrine stimulatory loop operating between the GHRH ligand and its SV1 receptor. In addition, tumors expressing SV1 might be direct targets for GHRH, present in systemic circulation. The present findings suggest that SV1-expressing tumors could be suitable targets for therapy based on GHRH antagonists. Future studies should be aimed at the identification of the subset of tumors that express significant levels of SV1 or other GHRH receptor isoforms with similar properties. The elucidation of the precise role of these truncated forms of the GHRH receptor in carcinogenesis should be of significant help in the development of new methods for therapy of various tumors based on GHRH antagonists.
Acknowledgments
We thank Dr. A. F. Parlow and the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Diseases, for RIA reagents, and Dr. Judit Horvath for editorial advice. This work was supported by the Medical Research Service of the Veterans Affairs Department, by a grant from Zentaris (Frankfurt, Germany) to Tulane University (to A.V.S.), and by National Institutes of Health Grant NS2608412 (to S.A.T.). Tulane University has applied for patents on some of the GHRH analogs cited in this paper. A.V.S. and J.L.V. are coinventors on those patents, but this paper deals with the mechanism of antitumor action of GHRH antagonists, which is a purely academic project.
Abbreviations
- GH
growth hormone
- GHRH
GH-releasing hormone
- IGF-I
insulin-like growth factor-I
- SV
splice variant
- SCLC
small cell lung carcinoma
- VIP
vasoactive intestinal peptide
References
- 1.Schally A V, Comaru-Schally A M. In: Cancer Medicine. 5th Ed. Holland J F, Frei E, II, Bast R C Jr, Kufe D W, Pollock R E, Weichselbaum R R, editors. New York: Dekker; 2000. pp. 715–729. [Google Scholar]
- 2.Pinski J, Schally A V, Groot K, Halmos G, Szepeshazi K, Zarandi M, Armatis P. J Natl Cancer Inst. 1995;87:1787–1794. doi: 10.1093/jnci/87.23.1787. [DOI] [PubMed] [Google Scholar]
- 3.Jungwirth A, Schally A V, Pinski J, Groot K, Armatis P, Halmos G. Proc Natl Acad Sci USA. 1997;94:5810–5813. doi: 10.1073/pnas.94.11.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halmos G, Schally A V, Varga J L, Plonowski A, Rekasi Z, Czompoly T. Proc Natl Acad Sci USA. 2000;97:10555–10560. doi: 10.1073/pnas.180313097. . (First Published August 29, 2000; 10.1073/pnas.180313097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jungwirth A, Schally A V, Pinski J, Halmos G, Groot K, Armatis P, Vadillo-Buenfil M. Br J Cancer. 1997;75:1585–1592. doi: 10.1038/bjc.1997.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamharzi N, Schally A V, Koppan M, Groot K. Proc Natl Acad Sci USA. 1998;95:8864–8868. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiaris H, Schally A V, Varga J L, Armatis P. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinski J, Schally A V, Jungwirth A, Groot K, Halmos G, Armatis P, Zarandi M, Vadillo-Buenfil M. Int J Oncol. 1996;9:1099–1105. doi: 10.3892/ijo.9.6.1099. [DOI] [PubMed] [Google Scholar]
- 9.Kiaris H, Schally A V, Varga J. Neoplasia. 2000;2:242–250. doi: 10.1038/sj.neo.7900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szepeshazi K, Schally A V, Groot K, Armatis P, Hebert F, Halmos G. Eur J Cancer. 2000;36:128–136. doi: 10.1016/s0959-8049(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 11.Szepeshazi K, Schally A V, Groot K, Armatis P, Halmos G, Hebert F, Szende B, Varga J L, Zarandi M. Br J Cancer. 2000;82:1724–1731. doi: 10.1054/bjoc.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahan Z, Varga J L, Schally A V, Rekasi Z, Armatis P, Chatzistamou I, Czompoly T, Halmos G. Breast Cancer Res Treat. 2001;60:71–79. doi: 10.1023/a:1006363230990. [DOI] [PubMed] [Google Scholar]
- 13.Chatzistamou I, Schally A V, Varga J L, Groot K, Busto R, Armatis P, Halmos G. Anti-Cancer Drugs. 2001;12:761–768. doi: 10.1097/00001813-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Szepeshazi K, Schally A V, Armatis P, Groot K, Hebert F, Feil A, Varga J L, Halmos G. Endocrinology. 2001;142:4371–4378. doi: 10.1210/endo.142.10.8426. [DOI] [PubMed] [Google Scholar]
- 15.Chatzistamou I, Schally A V, Varga J L, Groot K, Armatis P, Busto R, Halmos G. J Clin Endocrinol Metab. 2001;86:2144–2152. doi: 10.1210/jcem.86.5.7487. [DOI] [PubMed] [Google Scholar]
- 16.Schally A V, Varga J L. Trends Endocrinol Metab. 1999;10:383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 17.Kineman R D. Proc Natl Acad Sci USA. 2000;97:532–534. doi: 10.1073/pnas.97.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busto, R., Schally, A. V., Braczkowski, R., Plonowski, A., Krupa, M., Groot, K., Armatis, P. & Varga, J. L. (2001) Eur. J. Cancer, in press. [DOI] [PubMed]
- 19.Kahan Z, Arencibia J M, Csernus V J, Groot K, Kineman R D, Robinson W R, Schally A V. J Clin Endocrinol Metab. 1999;84:582–589. doi: 10.1210/jcem.84.2.5487. [DOI] [PubMed] [Google Scholar]
- 20.Frohman L A, Szabo M. Prog Clin Biol Res. 1981;74:259–271. [PubMed] [Google Scholar]
- 21.Schopohl J, Losa M, Frey C, Wolfram G, Huber R, Permanetter W, von Pawel J, Muller O A, von Werder K. Clin Endocrinol (Oxford, UK) 1991;34:463–467. doi: 10.1111/j.1365-2265.1991.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 22.Khorram O, Garthwaite M, Grosen E, Golos T. Fertil Steril. 2000;75:174–179. doi: 10.1016/s0015-0282(00)01658-7. [DOI] [PubMed] [Google Scholar]
- 23.Chopin L K, Herington A C. Prostate. 2001;49:116–121. doi: 10.1002/pros.1125. [DOI] [PubMed] [Google Scholar]
- 24.Plonowski, A., Schally, A. V., Busto, R., Krupa, M., Varga, J. L. & Halmos, G. (2001) Peptides, in press. [DOI] [PubMed]
- 25.Rekasi Z, Czompoly T, Schally A V, Halmos G. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. . (First Published August 29, 2000; 10.1073/pnas.180313297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izdebski J, Pinski J, Horvath J E, Halmos G, Groot K, Schally A V. Proc Natl Acad Sci USA. 1995;92:4872–4876. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rekasi Z, Varga J L, Schally A V, Halmos G, Groot K, Czompoly T. Proc Natl Acad Sci USA. 2000;97:1218–1223. doi: 10.1073/pnas.97.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 29.Bernhardt G, Reile H, Birnbock H, Sprufl T, Schonenberger H. J Cancer Res Clin Oncol. 1992;118:35–43. doi: 10.1007/BF01192309. [DOI] [PubMed] [Google Scholar]
- 30.Csernus V, Schally A V, Kiaris H, Armatis P. Proc Natl Acad Sci USA. 1999;96:3098–3103. doi: 10.1073/pnas.96.6.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer R E, Brinster R L, Rosenfeld M G, Evans R M, Mayo K E. Nature (London) 1985;315:413–416. doi: 10.1038/315413a0. [DOI] [PubMed] [Google Scholar]
- 32.Smit M J, Verzijl D, Iyengar R. Proc Natl Acad Sci USA. 1998;95:15084–15089. doi: 10.1073/pnas.95.25.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeAlmeida V I, Mayo K E. Mol Endocrinol. 1998;12:750–765. doi: 10.1210/mend.12.5.0102. [DOI] [PubMed] [Google Scholar]