Abstract
Antagonistic analogs of growth hormone-releasing hormone (GHRH) suppress growth of various tumors in vivo. This effect is exerted in part through inhibition of the GHRH–GH–insulin-like growth factor (IGF)-I axis. Nevertheless, because autocrine/paracrine control of proliferation by IGF-II also is a major factor in many tumors, the interference with this growth-stimulating pathway would offer another approach to tumor control. We thus investigated whether GHRH antagonists MZ-4-71 and MZ-5-156 also act on the tumor cells directly by blocking the production of IGF-II. An increase in the IGF-II concentration in the media during culture was found in 13 of 26 human cancer cell lines tested. Reverse transcription–PCR studies on 8 of these cell lines showed that they also expressed IGF-II mRNA. Antagonists of GHRH significantly inhibited the rate of proliferation of mammary (MDA-MB-468 and ZR-75–1), prostatic (PC-3 and DU-145), and pancreatic (MiaPaCa-2, SW-1990, and Capan-2) cancer cell lines as shown by colorimetric and [3H]thymidine incorporation tests and reduced the expression of IGF-II mRNA in the cells and the concentration of IGF-II secreted into the culture medium. Growth and IGF-II production of lung (H-23 and H-69) and ovarian (OV-1063) cancer cells that express mRNA for IGF-II and excrete large quantities of IGF-II also was marginally suppressed by the antagonists. These findings suggest that antagonistic analogs of GHRH can inhibit growth of certain tumors not only by inhibiting the GHRH–GH–IGF-I axis, but also by reducing the IGF-II production and by interfering with the autocrine regulatory pathway.
Insulin-like growth factors-I and -II (IGF-I and -II) are involved in the proliferation of various human cancers (1–4). Much evidence supports the view that growth hormone-releasing hormone–growth hormone (GHRH–GH)–IGF-I axis greatly influences the biologic behavior of many common neoplasms (2, 5–7). The tumor-promoting effect of IGFs has been shown in osteosarcomas and prostate cancers (8). Some tumor cells contain abundant IGF-I receptors and respond mitogenically to IGFs present in their microenvironment (6, 7). Activation of IGF receptors promotes growth of neuroectodermal tumors, neuroblastomas, choriocarcinomas, breast cancers, myelomas, and liver cancer (4–7, 9). mAb IR-3, which blocks IGF-I receptor function, inhibited growth of small-cell lung cancer, neuroblastoma, breast cancer, and rhabdomyosarcoma cells cultured in serum-free medium (10, 11). Local growth and metastatic behavior of IGF-I-responsive sarcomas are reduced by hypophysectomy and restored by administration of growth hormone (12, 13). It has been shown that growth of breast cancer was significantly reduced in mice with a genetically suppressed GH–IGF-I axis (14).
These investigations (1–14) and others support the hypothesis that aggressive behavior of many neoplasms may be reduced by interfering with the GHRH–GH–IGF-I axis. One of the pharmacological approaches aimed at achieving an inhibition could be based on utilization of antagonistic analogs of GHRH. GHRH antagonists developed by us (2, 15, 16) inhibit the growth of human renal adenocarcinoma, prostate cancers and small-cell and non-small-cell lung carcinomas, osteosarcomas, and other tumors xenografted into nude mice (17–21). It was suggested that suppressive effects of antagonistic analogs of GHRH on tumor growth in vivo could be caused in part by a reduction in pituitary GH release and the subsequent decrease in production of IGF-I in the liver (2, 21). However, the reduction in serum IGF-I level did not always parallel tumor suppression and tumor levels of IGF-I and IGF-II in renal cancers, lung cancers, and prostate cancers were greatly inhibited after therapy with GHRH antagonists (17–20). This observation suggests that, in addition to hepatic IGF-I, the inhibitory effect of antagonistic analogs of GHRH on tumor growth may be mediated by regulation of tumor levels of IGF-I and -II.
The serum level of IGF-II, unlike that of IGF-I, is independent of the functional state of GHRH–GH axis. Unlike IGF-I, which is mostly produced in the liver, IGF-II is synthesized by a wide variety of tissues. IGF-II is considered one of the key cell-survival factors (22), and its secretion is controlled primarily by the local environment of the cells. The observation that certain tumor cells proliferate in the absence of serum-derived growth factors gave rise to the idea that such cells are capable of secreting their own growth factors. Various studies demonstrated IGF-II production and expression of IGF-II mRNA in several tumor-cell lines especially in diverse sarcomas and neural tumors (1, 23, 24). The presence of receptors for both IGF-I and IGF-II also was shown in several tumor cells (1, 2, 10, 23, 25). These studies provide evidence that IGFs produced by these cells may play a fundamental role in their proliferation.
Autocrine/paracrine regulatory mechanisms involving IGF-II are implicated in proliferation of normal tissues as liver, colon, lung, or bone and also participate in nerve regeneration and wound healing (1, 4, 26). IGF-II also affects growth of various tumors like neuroblastomas, chondrosarcomas, Wilms tumor, mesothelial tumors, and cancers of breast, colon, prostate, endometrium, and liver in autocrine/paracrine fashion (1, 2, 4, 27–33). Interrupting the autocrine regulatory circle of IGF-II could provide an efficacious approach to inhibiting various cancers. In addition to blocking the function of the IGF receptors on the surface of tumor cells, this goal also can be achieved by reducing IGF-II production of the cells. The mechanism of the control of IGF-II production in tumor cells, however, has not been elucidated so far.
Besides the hypothalamus, GHRH also is produced in various peripheral tissues including tumors (34, 35). The receptors for GHRH also were detected in various extrapituitary organs (36). These results suggest that another mechanism of the tumor growth-suppressing effect of the antagonistic analogs of GHRH could be based on blocking the autocrine regulatory pathway of IGF-II directly in the tumor cells or in their immediate environment. Thus, the goal of this study was to clarify whether the antagonistic analogs for GHRH can interfere with the autocrine stimulatory function of IGF-II in tumor cells. To exclude the participation of the GHRH–GH–IGF-I axis operating in vivo, we designed in vitro experiments. Cancer cells of human origin were studied in culture and the effects of antagonistic analogs of GHRH on growth, IGF-II production, and expression of IGF-II mRNA were evaluated.
MATERIALS AND METHODS
Peptides.
GHRH antagonists [Ibu-Tyr1,d-Arg2,Phe(4-Cl)6,Abu15,Nle27,Agm29]hGHRH(1–29) (MZ-4–71) and [PhAc-Tyr1,d-Arg2,Phe(4-Cl)6,Abu15,Nle27,Agm29]hGHRH(1–29) (MZ-5-156) and hGHRH(1–29), used as a standard for in vitro experiments, were synthesized and characterized in our laboratory as reported (15, 16). Other organic and inorganic chemicals were purchased from Sigma.
Tissue Cultures.
Tumor cell lines were obtained from the American Type Culture Collection. The media for routine culture (GIBCO/BRL) varied depending on the cell line. The type of tissue culture medium varied according to the requirements of the cell lines: RPMI medium 1640 (RPMI) + 10% fetal bovine serum (FBS) were used for Capan-2, DU-145, H-23, H-69, JAR, HEC-1A, and LNCaP cells; RPMI + 5% FBS for H-345 and PC-3 cells; RPMI + 10% newborn calf serum (NCS) for H-157 and H-510 cells; McCoy 5A Medium + 10% FBS for HT-29 and SKOV-3 cells; F12 + 20% FBS for LoVo cells; improved minimal essential medium (IMEM) + dextran-coated charcoal-treated FBS for MCF-7 cells; DMEM + 10% NCS for MDA-MB-231 cells; IMEM + 10% FBS for MDA-MB-468 cells; DMEM + 10% FBS for Panc-1 cells; L15 + 10% FBS for SW-1990 cells; RPMI + 10% FBS supplemented with insulin for T47D cells; minimal essential medium (MEM) + 10% FBS supplemented with pyruvate for U373MG cells; RPMI + 10% FBS supplemented with pyruvate and glucose for ZR-75–1 cells; and RPMI + 10% FBS + pyruvate and MEM vitamins for OV-1063 cells. The cultures were maintained in a humidified atmosphere containing 5% CO2/95% air at 37°C. The cells were passaged weekly and routinely monitored for the presence of mycoplasma by using a test kit from Boehringer Mannheim.
Colorimetric Tests.
Crystal violet assay was performed as described (37). The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] test is based on a method described by Plumb (38) and was carried out as previously reported (18, 20, 21).
[3H]Thymidine Incorporation Test.
Cells were seeded into 96-well microplates in the appropriate medium. When cells were 50% confluent (adherent cells) or after 24 hours (suspension cells), the compounds to be tested and control media were added, and the plates were incubated under standard conditions for another 20 hours. All subsequent steps were performed as described (17, 21).
Radioimmunoassay of IGF-II.
Because only tissue culture media of defined composition were used, the acid–ethanol extraction method to eliminate IGF-binding proteins was unnecessary. The radioimmunoassay for IGF-II was carried out as reported (17–20).
RNA Extraction.
Harvested tumor cells were washed once with PBS, and total RNA was isolated by using the RNAzol B reagent (Tel-Test, Friendswood, TX) following the manufacturer’s instructions.
Reverse Transcription (RT).
One microgram of total RNA was reverse-transcribed into cDNA by using Moloney murine leukemia virus reverse transcriptase according to manufacturer’s instructions (Perkin–Elmer).
PCR Amplification.
One microliter of the cDNA was amplified in a 50-μl solution containing 10 mM Tris⋅HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM of each dNTP, 2.5 units of Taq DNA polymerase (Perkin–Elmer) and 0.4 μM each primer. The primers used were 5′-TCCTCTGACTTCAACAGCGACACC-3′ (sense) and 5′-TCTCTCTTCCTCTTGTGCTCTTGG-3′ (antisense) for hGAPDH and 5′-AGTCGATGCTGGTGCTTCTCACCTTCTTGGC-3′ (sense) and 5′-TGCGGCAGTTTTGCTCACTTCCGATTGCTGG-3′ (antisense) for IGF-II (19). PCR consisted of 1 cycle at 95°C for 3 min, 62°C for 1 min, and 72°C for 1 min and subsequently 26 cycles for GAPDH or 26 to 33 cycles for IGF-II, depending on the endogenous levels of expression, at 95°C for 35 sec, 62°C for 40 sec, and 72°C for 40 sec. The reactions were performed by using a Stratagene Robocycler 40 system (Stratagene). The number of cycles for each cell line was determined in preliminary experiments to be within the exponential range of PCR amplification (data not shown). Five microliters of each PCR product was separated by electrophoresis in 2% agarose gel and stained with ethidium bromide, or for quantitative analysis, in 8% polyacrylamide gel and stained with silver. The intensity of the bands was analyzed by a scanning densitometer (Model GS-700, Bio-Rad) coupled with the Bio-Rad PC analysis software.
Statistical Analysis.
For statistical analysis, two-tailed Student’s t test was used. The difference was considered significant at P < 0.05 tail probability.
RESULTS
IGF-II Production of Cancer Cell Lines.
Twenty-six human cancer cell lines were grown in vitro until they covered ≈70% of the surface of the culture dish, the experiment lasting for 3 to 7 days. IGF-II concentration of samples from the culture media taken before and after the culture period was determined by radioimmunoassay (Table 1). Because of wide variations in experimental conditions, including the rate of cell proliferation, type of medium, form of cell growth—monolayer or floating cell-clusters—only semiquantitative data are given (Table 1). Most of the media contained IGF-II (5–15 ng/ml) at the beginning of the experiments because of their bovine serum content. IGF-II production of the cells was considered negative, if IGF-II concentration of the sample taken at the end of the experiment was similar to or lower than that at the beginning.
Table 1.
Semiquantitative evaluation of the IGF-II production (increase in the concentration of IGF-II in the medium) of some human cancer cell lines in tissue culture
| Cancer cell line | Increase by 2–12 ng/ml | Increase by <2 ng/ml | No increase |
|---|---|---|---|
| Breast | MDA-MB-468 | ZR-75-1 | MCF-7-M-III† MDA-MB-231 MCF-7-LCC1† T47D |
| Prostatic | PC-3 | DU-145 | LNCaP |
| Lung | |||
| SCLC | H-69 | H-510 | H-345† |
| non-SCLC | H-23 | H-157† | |
| Pancreatic | MiaPaCa-2 SW-1990 | Capan-2 | Panc-1† |
| Ovarian | OV-1063 SKOV-3 | ||
| Colorectal | LoVo | HT-29 | |
| Choriocarcinoma | JAR† | ||
| Osteosarcoma | MG-63 | ||
| Glioma | U-373-MG | ||
| Endometrial | HEC-1A | ||
| Total | 6 | 7 | 13 |
The cell lines were grouped according to the increase in the amount of IGF-II in the medium. The incubation time varied from 2 to 5 days depending on the rate of proliferation of the cells.
Cell lines showing a decrease in IGF-II content in the medium.
SCLC, small-cell lung carcinoma; non-SCLC, non-small-cell lung carcinoma.
Thirteen of the twenty-six cell lines showed an increase in IGF-II concentration. In six cell lines, the increase was substantial (2–12 ng/ml). In some cell lines, a considerable decrease in IGF-II content of the medium was found (Table 1). Subsequent experiments described below then were performed with tumor cells found to secrete IGF-II.
Effect of Antagonistic Analogs of GHRH on IGF-II Release from Human Cancer Cell Lines.
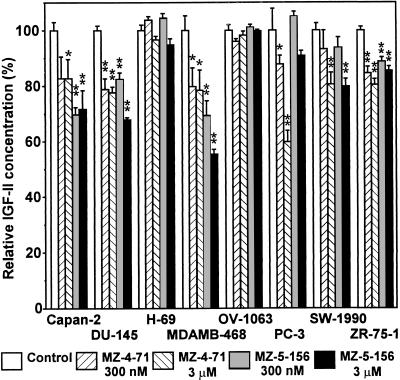
Precultured human cancer cells were incubated in seeding medium for 24–48 hours and (at time T0) the medium was replaced by test medium. Each of the cell lines was divided into five groups. One group of cells served as a control and the other four groups were incubated in medium containing MZ-4-71 or MZ-5-156 at 300 nM and 3 μM concentrations for 42–97 hours (T1). The actual duration of each experiment depended on the growth rate of the respective cell-line. IGF-II content of media at T0 and T1 times was determined. Changes in IGF-II concentrations as compared with those of the respective control groups are presented in Fig. 1. GHRH analogs induced a significant decrease in IGF-II concentrations in most of the cell lines tested. DU-145 prostate cancer cells and MDA-MB-468 and ZR-75–1 breast cancer cells responded to lower (300 nM) concentrations of both analogs. Capan-2 pancreatic cancer was more sensitive to MZ-5-156, whereas PC-3 prostate cancer responded better to MZ-4–71. IGF-II secretion of SW-1990 pancreatic carcinoma was inhibited significantly only by higher (3 μM) concentration of the analogs. H-69 small-cell lung carcinoma and OV-1063 ovarian cancer did not respond to the analogs under the experimental conditions.
Figure 1.
The effect of antagonistic analogs of GHRH (MZ-4-71 and MZ-5-156) on IGF-II production of human cancer cell lines in culture. Each group consisted of 4–8 wells with 3,000–5,000 cells per well. The cells were exposed to the analogs at 300 nM or 3 μM concentration for 42–97 hours depending on the rate of proliferation of the cell line. Control cultures received medium alone. Changes of IGF-II concentration in the tissue culture media as compared with control groups are plotted as mean ± SEM of 4–6 experiments. Significant differences from the control groups are indicated: ∗, 0.05 > P > 0.01; ∗∗, P < 0.01, two-tailed Student’s t test.
Effect of GHRH Antagonists on the Rate of Proliferation of Cancer Cell Lines.
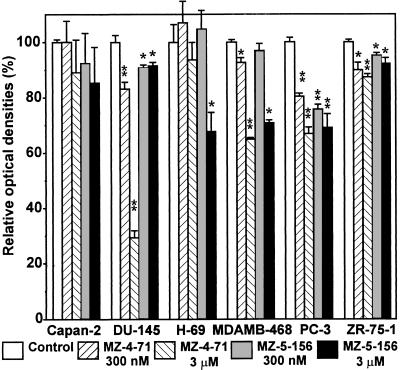
In experiments similar to those described above, we tested the effect of the antagonistic analogs of GHRH on the proliferation of the cancer cells. At the beginning (T0) and end (T1) of the incubation periods, colorimetric tests were performed. In the case of H-69 cells that mostly float in culture, the thiazolyl-blue (MTT) test was carried out, but for the majority of the cells that attach well to the surface of the wells, crystal violet tests were done. The differences in optical densities measured at T1 and T0 times for cell groups treated with the analog compared with those of respective controls are shown in Fig. 2. As can be seen, the analogs decreased the growth of most of the cancer cell lines tested. The reduction in growth was significant at both doses of analogs in the case of DU-145 and PC-3 prostate cancers and ZR-75-1 and MDA-MB-468 breast cancers (except for the lower concentration of MZ-5-156). In these experiments, the growth of H-69 small-cell lung carcinoma was inhibited only at high concentration of MZ-5-156, and the growth reduction of Capan-2 pancreatic cancer was not significant.
Figure 2.
The effects of antagonistic analogs of GHRH on growth of human cancer cells as determined by colorimetric tests (MTT for H-69 and crystal violet for other cells). The experimental conditions were similar to those in Fig. 1. ∗, 0.05 > P > 0.01; ∗∗, P < 0.01, two-tailed Student’s t test.
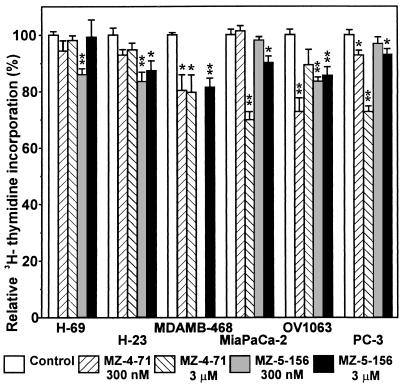
The effect of the analogs of GHRH antagonists on the proliferation of various human cancer cells also was investigated by using [3H]thymidine incorporation assay. The experimental design was similar to those described above. Groups of cancer cells were exposed to MZ-4-71 or MZ-5-156 at 300 nM and 3 μM concentrations for 24 hours and 0.2 μCi [3H]thymidine (1 Ci = 37 GBq) was then added to each well. After 4 hours exposure time, the cells were washed and their radioactivities were counted. The incorporation of radioactivity into the cells treated with analog was compared with that of the control group. The results are shown in Fig. 3. Antagonistic analogs of GHRH significantly reduced the incorporation of [3H]thymidine into DNA in MDA-MB-468 breast cancers, OV-1063 ovarian cancers (except for 3 μM MZ-4–71), and PC-3 prostate cancers (except for 300 nM MZ-5-156). In MiaPaCa-2 pancreatic cancer, the inhibition occurred only at higher doses of both analogs. In the case of lung carcinoma cells (H-69 and H-23), only MZ-5-156 was active.
Figure 3.
The effects of antagonistic analogs on [3H]thymidine incorporation into DNA of cultured human cancer cell lines. The cells were exposed to MZ-4-71 or MZ-5-156 for 24 hours, [3H]thymidine was added, and the incubation was continued for 4 hours. Relative [3H] activities of the washed cells (mean ± SEM) are plotted relative to those of control groups. Significant differences from the control groups are indicated. ∗, 0.05 > P > 0.01; ∗∗, P < 0.01, two-tailed Student’s t test.
Expression of IGF-II mRNA in Cancer Cell Lines.
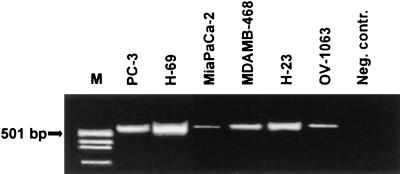
mRNA extracted from various cultured cancer cells, including MDA-MB-468 breast cancer, PC-3 prostate cancer, MiaPaCa-2 pancreatic cancer, OV-1063 ovarian cancer, and H-23 and H-69 lung cancer cells was reverse-transcribed, and IGF-II and hGAPDH cDNA was amplified by using RT-PCR (31 and 26 cycles, respectively). The product was separated by electrophoresis in 2% agarose and stained with ethidium bromide (Fig. 4) or in 8% polyacrylamide and stained with silver. All of the cells tested expressed IGF-II mRNA. The intensities of the silver-stained bands were analyzed with a computer-controlled densitometer. From the relative intensities (mRNA of IGF-II normalized for mRNA of GAPDH), semiquantitative results on the levels of IGF-II mRNA were obtained (data not shown). The highest expression of IGF-II mRNA was shown by lung carcinoma cells (H-69 and H-23), followed by MDA-MB-468 breast cancer and PC-3 prostate cancer (Fig. 4).
Figure 4.
IGF-II mRNA expression in cultured human cancer cell lines. mRNA from each cell line was amplified by using RT-PCR. After electrophoresis in 2% agarose gel, the product was stained with ethidium bromide. The PCR product was of the expected size (538 bp) for IGF-II. M, pUC18/MspI-digested cDNA marker.
Effect of GHRH Antagonists MZ-4-71 and MZ-5-156 on Expression of IGF-II mRNA in Tumor Cells.
Tumor cells of human origin were cultured for 4 hours in the presence of 3 μM antagonists MZ-4-71 or MZ-5-156. Control cultures received medium alone. The cells were then harvested, and their RNA was extracted. IGF-II mRNA was amplified by using RT-PCR. After electrophoresis on polyacrylamide gel, optical densities of the silver-stained bands were measured and the levels of IGF-II mRNA were assessed by semiquantitative RT-PCR. Optical densities of IGF-II cDNA bands (corrected by the density of the hGAPDH band) relative to those of the untreated controls, which were arbitrarily considered as 100%, are shown in Table 2. As can be seen, antagonistic analogs of GHRH substantially reduced the levels of IGF-II mRNA in MDA-MB-468 breast cancers, PC-3 prostate cancers, MiaPaCa-2 pancreatic cancers, and OV-1063 ovarian cancers (MZ-5-156 only). Neither analog showed a significant effect on IGF-II mRNA levels in H-23 and H-69 lung cancer cells.
Table 2.
The effect of antagonistic analogs of GHRH on IGF-II mRNA expression of human cancer cell lines
| Cell lines | Control, % | MZ-4-71, % | MZ-5-156, % |
|---|---|---|---|
| Capan-2 | 100 | 83 | 65 |
| H-23 | 100 | 93 | 91 |
| H-69 | 100 | 95 | 96 |
| MiaPaCa-2 | 100 | 80 | 70 |
| MDA-MB-468 | 100 | 59 | 58 |
| OV-1063 | 100 | 98 | 73 |
| PC-3 | 100 | 70 | 77 |
| SW-1990 | 100 | 92 | 86 |
The cells were exposed to 3 μM concentration of analogs MZ-4-71 or MZ-5-156 for 4 hours followed by semiquantitative multiplex RT-PCR and electrophoresis on polyacrylamide gel. Optical densities of silver-stained IGF-II and hGAPDH cDNA bands were measured. Data for IGF-II bands were corrected by their hGAPDH values. Relative corrected IGF-II band densities compared to those of the control group are given in %.
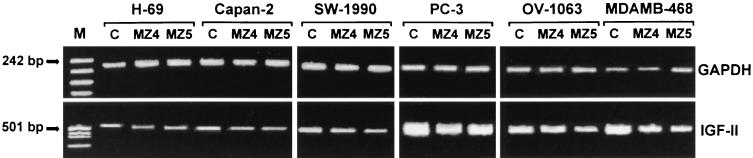
In a similar experiment, electrophoresis of RT-PCR product was performed on 2% agarose gel, and the bands were stained with ethidium bromide (Fig. 5). The results were similar to those obtained in the previous experiment. In this case, a visible reduction in the density of IGF-II cDNA bands of the Capan-2 and SW-1990 pancreatic cancer cells treated with GHRH antagonists could also be observed.
Figure 5.
The effect of antagonistic analogs of GHRH on IGF-II mRNA expression in cultured human cancer cell lines. The cells were exposed to the analogs (MZ4 = MZ-4-71, MZ5 = MZ-5-156) at 3 μM concentration for 4 hours. mRNA was extracted from the cells and amplified by using RT-PCR. After electrophoresis in 2% agarose gel, the product was stained with ethidium bromide. For internal control, GAPDH-mRNA was obtained and visualized in the same way from the RNA extracts. The PCR products were of the expected size (538 bp for IGF-II and 207 bp for GAPDH). C, control cells; M, pUC18/MspI-digested cDNA marker.
DISCUSSION
Malignant tumors are among the leading causes of death in western countries. Because of the heterologous nature of many malignancies, the treatment of cancer requires a variety of approaches aimed at different elements of the intracellular machinery responsible for cell growth. Some strategies can be directed at the signaling pathways controlled by growth factors that are necessary for the proliferation of most cancers. IGF-I and IGF-II are among the key growth factors involved in tumor growth (1–7). A reduction in the concentration of IGF-I or IGF-II in the microenvironment of the tumor cells could decrease the growth of various tumors. Because IGF-I is mostly produced by the liver and is under the control of the GHRH–pituitary GH axis, serum concentration of IGF-I can be substantially reduced by inhibition of GH release (2, 5, 19). In contrast, IGF-II is produced by a wide variety of cells controlled by unknown factors and is considered to exert mostly paracrine or autocrine effect (1, 2, 4, 24, 32).
Antagonistic analogs of GHRH developed in our laboratory appear to be excellent candidates for inhibiting tumor growth by suppressing the GHRH–GH–IGF-I axis (2, 15, 16). We have already shown that analogs such as MZ-4-71 and MZ-5-156 can inhibit growth of various tumors both in vivo and in vitro (17–21). These GHRH antagonists reduced IGF-I concentration in serum, but their effects and in vitro activity could not be explained merely by the suppression of hepatic IGF-I production (17–21). This conclusion led to the demonstration that in vivo these analogs also influence the local IGF-II production in tumors (17–20). To assess the involvement of the antagonists in blocking the IGF-II autocrine regulatory pathway in tumor cells, in vitro experiments were performed. Previous studies reported IGF-II production in several tumors including sarcomas, adrenal, kidney, or brain tumors, as well as pancreatic and prostatic cancers (1, 4, 23, 24, 27, 28). To extend this list, we tested 26 human cancer cell lines for IGF-II production in vitro. Six of these cell lines, including MDA-MB-468, PC-3, H-69, H-23, MiaPaCa-2 and SW-1990, showed a major and 7 showed a moderate increase in IGF-II levels in the culture medium, indicating IGF-II production by these cells (Table 1). Most of the cells tested could be cultured only in the presence of bovine serum containing significant quantities (5–15 ng/ml) of IGF-II. In some cases, a considerable decrease in IGF-II concentration was found during the culture period. This may indicate enzymatic inactivation or cellular uptake of IGF-II originally present in the medium. The difference between the IGF-II contents present in the medium before and after the culture period is a function of two factors, which by themselves were not measurable in our experimental conditions—the clearance of IGF-II originally present in the medium and production of IGF-II by the cells. An increase in IGF-II concentration definitely indicates IGF-II production by the cells, whereas no increase or even a slight decrease may still occur in the case of a modest IGF-II release accompanied by an extensive reduction in IGF-II because of inactivation or cellular uptake. This is why no numerical data were given in Table 1.
Some of the cell lines, including MDA-MB-468, PC-3, MiaPaCa-2, H-69, H-23, OV-1063, Capan-2, and SW-1990, that release IGF-II into the tissue culture medium, also were tested for the expression of IGF-II mRNA. All of these cells showed different degrees of IGF-II mRNA expression. The results are in good agreement with the measurement of IGF-II release into the medium. The detection of an increase in IGF-II in the media showed that IGF-II mRNA not only was expressed in certain tumor cells, but also was translated and secreted from the cells.
Antagonistic analogs of GHRH, MZ-4-71, and MZ-5-156, which proved to be potent inhibitors of tumor growth in vivo, also were active in inhibiting growth, IGF-II release, and expression of IGF-II mRNA of MDA-MB-468 and ZR-75-1 breast cancers, PC-3 and DU-145 prostatic cancers, and MiaPaCa-2, Capan-2, and SW-1990 pancreatic cancers in vitro. Although H-69 and H-23 lung and OV-1063 ovarian cancer cells synthesize and secrete high quantities of IGF-II, their growth and IGF-II production were only marginally affected by these analogs.
Our results indicate that antagonistic analogs of GHRH reduce the concentrations of IGF-II in the microenvironment of the tumor cells, at least in the case of some breast, prostate, and pancreatic cancers. As has been reported previously, these analogs reduce the blood level of IGF-I by inhibiting GH release from the anterior pituitary (17–21). The present study provides evidence that in the case of several cancers, these analogs also interrupt the autocrine regulatory pathway of IGF-II produced by the tumor cells. Because IGFs are necessary for proliferation of most tumor cells, the antagonistic analogs of GHRH appear to be potential candidates for therapy of various tumors.
Much evidence exists that octapeptide analogs of somatostatin such as Sandostatin (Octreotide) and RC-160 (Vapreotide) suppress GH release, lower the levels of hepatic IGF-I, and, thus, reduce the proliferation of some IGF-I-responsive neoplasms (reviewed in ref. 5). The inhibition of hepatic IGF-I gene expression by Octreotide has also been reported (5). However, analogs of somatostatin show selectivity for subtypes 2 and 5 of somatostatin receptors (SSTR-2 and -5), which are not expressed by a significant subset of tumors (5). In the absence of these receptors, somatostatin octapeptide analogs cannot exert major direct effects. Moreover, there is so far no evidence that somatostatin analogs can suppress tumor levels of IGF-I and IGF-II and expression of mRNAs of IGF-I and IGF-II in tumors.
The results presented in this paper indicate that, in some human cancer cell lines, the antagonistic analogs of GHRH inhibit proliferation of tumor cells by interfering with the autocrine regulatory effect of IGF-II. However, the mechanism of action of GHRH antagonists remains to be clarified. It was shown that several human cancers and cancer cell lines produce GHRH (34, 35, 39). GHRH-sensitive receptors on various tumor cells have been also described (19). Thus, the GHRH antagonists may block these receptors that could be responsible for controlling the IGF-II production of the cells. Although the decrease in IGF-II expression and production was relatively small under our experimental conditions, the biological importance of these findings is supported by the observation that the levels of IGF-II mRNA decreased after a short exposure of only 4 hr to the GHRH antagonists. In addition, the fact that MZ-4-71 and MZ-5-156 showed slightly different potencies in inhibiting growth of various tumor cells in vitro may indicate that different receptors or receptor subtypes could be present in various tumors, providing further support for the specificity of these findings.
Acknowledgments
The authors thank Dr. Kate Groot for performing the radioummunoassay for IGF-II. This work was supported by the Medical Research Service of the Veterans Affairs Department and CaP CURE (Association for the Cure of Prostate Cancer) and by a grant from ASTA Medica (Frankfurt am Main, Germany) to Tulane University School of Medicine (all to A.V.S.).
ABBREVIATIONS
- GH
growth hormone
- GH-RH
GH-releasing hormone
- IGF-I
insulin-like growth factor-I
- IGF-II
insulin-like growth factor-II
- RT
reverse transcription
References
- 1.Toretsky J A, Helman L J. J Endocrinol. 1996;149:367–372. doi: 10.1677/joe.0.1490367. [DOI] [PubMed] [Google Scholar]
- 2.Schally A V, Kovacs M, Toth K, Comaru-Schally A M. In: Growth Hormone Secretagogues in Clinical Practice. Bercu B B, Walker R F, editors. New York: Dekker; 1998. pp. 145–162. [Google Scholar]
- 3.Westley B R, May F E B. Br J Cancer. 1995;72:1065–1066. doi: 10.1038/bjc.1995.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daughaday W H. Endocrinology. 1990;127:1–4. doi: 10.1210/endo-127-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Pollak M N, Schally A V. Proc Soc Exp Biol Med. 1998;217:143–152. doi: 10.3181/00379727-217-44216. [DOI] [PubMed] [Google Scholar]
- 6.Macaulay V M. Br J Cancer. 1992;65:311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullen K J, Yee D, Rosen N. Cancer Invest. 1991;9:443–454. doi: 10.3109/07357909109084643. [DOI] [PubMed] [Google Scholar]
- 8.Ritchie C K, Andrews L R, Thomas K G, Tindall D J, Fitzpatrick L A. Endocrinology. 1997;138:1145–1150. doi: 10.1210/endo.138.3.4974. [DOI] [PubMed] [Google Scholar]
- 9.Singleton J R, Randolph A E, Feldman E L. Cancer Res. 1996;56:4522–4529. [PubMed] [Google Scholar]
- 10.Nakanishi Y, Mulshine J L, Kasprzyk P G, Natale R B, Maneckjee R, Avis I, Treston A M, Gazdar A F, Minna J D, Cuttitta F. J Clin Invest. 1988;82:354–359. doi: 10.1172/JCI113594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arteaga C L, Kitten L J, Coronado E B, Jacobs S, Kull F C, Jr, Allred D C, Osborne C K. J Clin Invest. 1989;84:1418–1423. doi: 10.1172/JCI114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollak M, Sem A W, Richard M, Tetenes E, Bell R. J Natl Cancer Inst. 1992;84:966–971. doi: 10.1093/jnci/84.12.966. [DOI] [PubMed] [Google Scholar]
- 13.Sekyi-Otu A, Bell R, Andrulis I, Pollak M. J Natl Cancer Inst. 1994;86:628–632. doi: 10.1093/jnci/86.8.628. [DOI] [PubMed] [Google Scholar]
- 14.Yang X F, Beamer W G, Huynh H, Pollak M. Cancer Res. 1996;56:1509–1511. [PubMed] [Google Scholar]
- 15.Zarandi M, Horvath J E, Halmos G, Pinski J, Nagy A, Groot K, Rekasi Z, Schally A V. Proc Natl Acad Sci USA. 1994;91:12298–12302. doi: 10.1073/pnas.91.25.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarandi M, Kovacs M, Horvath J E, Toth K, Halmos G, Groot K, Nagy A, Kele Z, Schally A V. Peptides. 1994;18:423–430. doi: 10.1016/s0196-9781(96)00344-0. [DOI] [PubMed] [Google Scholar]
- 17.Jungwirth A, Schally A V, Pinski J, Groot K, Armatis P, Halmos G. Proc Natl Acad Sci USA. 1997;94:5810–5813. doi: 10.1073/pnas.94.11.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jungwirth A, Schally A V, Pinski J, Halmos G, Groot K, Armatis P, Vadillo-Buenfil M. Br J Cancer. 1997;75:1585–1592. doi: 10.1038/bjc.1997.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamharzi N, Schally A V, Koppán M, Groot K. Proc Natl Acad Sci USA. 1998;95:8864–8868. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinski J, Schally A V, Jungwirth A, Groot K, Halmos G, Armatis P, Zarandi M, Vadillo-Buenfil M. Int J Oncol. 1996;9:1099–1105. doi: 10.3892/ijo.9.6.1099. [DOI] [PubMed] [Google Scholar]
- 21.Pinski J, Schally A V, Groot K, Halmos G, Szepeshazi K, Zarandi M, Armatis P. J Natl Cancer Inst. 1995;87:1787–1794. doi: 10.1093/jnci/87.23.1787. [DOI] [PubMed] [Google Scholar]
- 22.Lamm G M, Christofori G. Cancer Res. 1998;58:801–807. [PubMed] [Google Scholar]
- 23.Daughaday W H, Emanuele M A, Brooks M H, Barbato A L, Kapadia M, Rotwein P. N Engl J Med. 1988;319:1434–1440. doi: 10.1056/NEJM198812013192202. [DOI] [PubMed] [Google Scholar]
- 24.Angelloz-Nicoud P, Binoux M. Endocrinology. 1995;136:5485–5492. doi: 10.1210/endo.136.12.7588299. [DOI] [PubMed] [Google Scholar]
- 25.Lee P D, Rosenfeld R G, Hintz R L, Smith S D. J Clin Endocrinol Metab. 1986;62:28–35. doi: 10.1210/jcem-62-1-28. [DOI] [PubMed] [Google Scholar]
- 26.Stiles A D, D’Ercole A J. Am J Respir Cell Mol Biol. 1990;3:93–100. doi: 10.1165/ajrcmb/3.2.93. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt S, Ren-Qiu Q, Torresani T, Doebeli M, Zapf J, Schoenle E J. Eur J Endocrinol. 1997;137:396–401. doi: 10.1530/eje.0.1370396. [DOI] [PubMed] [Google Scholar]
- 28.Leventhal P S, Randolph A E, Vesbit T E, Schenone A, Windebank A, Feldman E L. Exp Cell Res. 1995;221:179–186. doi: 10.1006/excr.1995.1365. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y Q, Grundy P, Polychronakos C. Oncogene. 1997;14:1041–1046. doi: 10.1038/sj.onc.1200926. [DOI] [PubMed] [Google Scholar]
- 30.Bentel J M, Lebwohl D E, Cullen K J, Rubin M S, Rosen M, Mendelsohn J, Miller W H., Jr J Cell Physiol. 1995;165:212–221. doi: 10.1002/jcp.1041650124. [DOI] [PubMed] [Google Scholar]
- 31.Hershkovitz E, Marbach M, Bosin E, Levy J, Roberts C T, Jr, LeRoith D, Schally A V, Sharoni Y. J Clin Endocrinol Metab. 1993;77:963–968. doi: 10.1210/jcem.77.4.8408472. [DOI] [PubMed] [Google Scholar]
- 32.Figueroa J A, Lee A V, Jackson J G, Yee D. J Clin Endocrinol Metab. 1995;80:3476–3482. doi: 10.1210/jcem.80.12.8530586. [DOI] [PubMed] [Google Scholar]
- 33.Kleinman D, Roberts C T, Jr, LeRoith D, Schally A V, Levy J, Sharoni Y. Regul Pept. 1993;48:91–98. doi: 10.1016/0167-0115(93)90338-9. [DOI] [PubMed] [Google Scholar]
- 34.Frohman L A, Thominet J L, Szabo M. In: Human Growth Hormone. Raiti S, Tolman R A, editors. New York: Plenum; 1986. pp. 347–360. [Google Scholar]
- 35.Benlot C, Levy A, Fontanaud P, Roche A, Joubert D. J Clin Endocrinol Metab. 1997;82:690–696. doi: 10.1210/jcem.82.2.3754. [DOI] [PubMed] [Google Scholar]
- 36.Mayo K E, Godfrey P A, Suhr S T, Kulik D J, Rahal J O. Recent Prog Horm Res. 1995;50:35–73. doi: 10.1016/b978-0-12-571150-0.50007-x. [DOI] [PubMed] [Google Scholar]
- 37.Reile H, Birnbock H, Bernhardt G, Spruss T, Schonenberger H. Anal Biochem. 1990;187:262–267. doi: 10.1016/0003-2697(90)90454-h. [DOI] [PubMed] [Google Scholar]
- 38.Plumb J A, Milroy R, Kaye S B. Cancer Res. 1989;49:4435–4440. [PubMed] [Google Scholar]
- 39.Kahán Z, Arencibia J M, Csernus V J, Groot K, Kineman R D, Robinson W R, Schally A V. J Clin Endocrinol Metab. 1999;84:582–590. doi: 10.1210/jcem.84.2.5487. [DOI] [PubMed] [Google Scholar]