Abstract
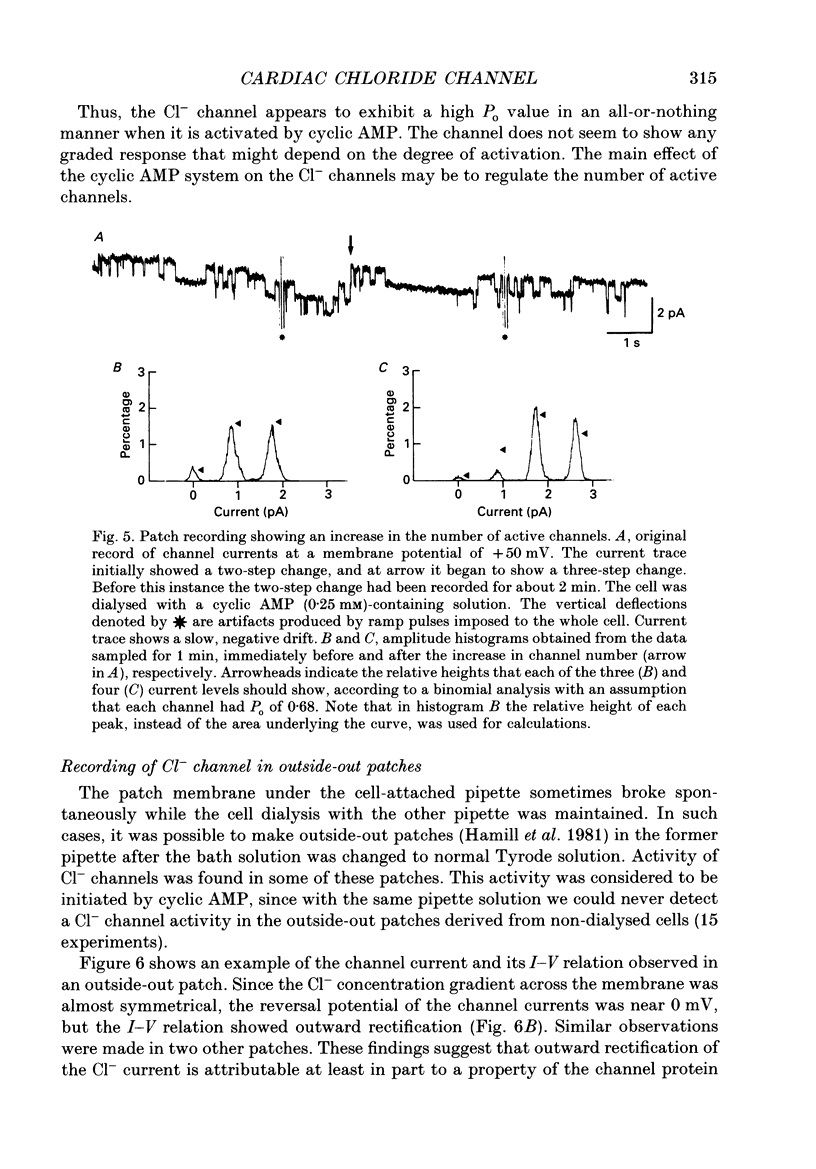
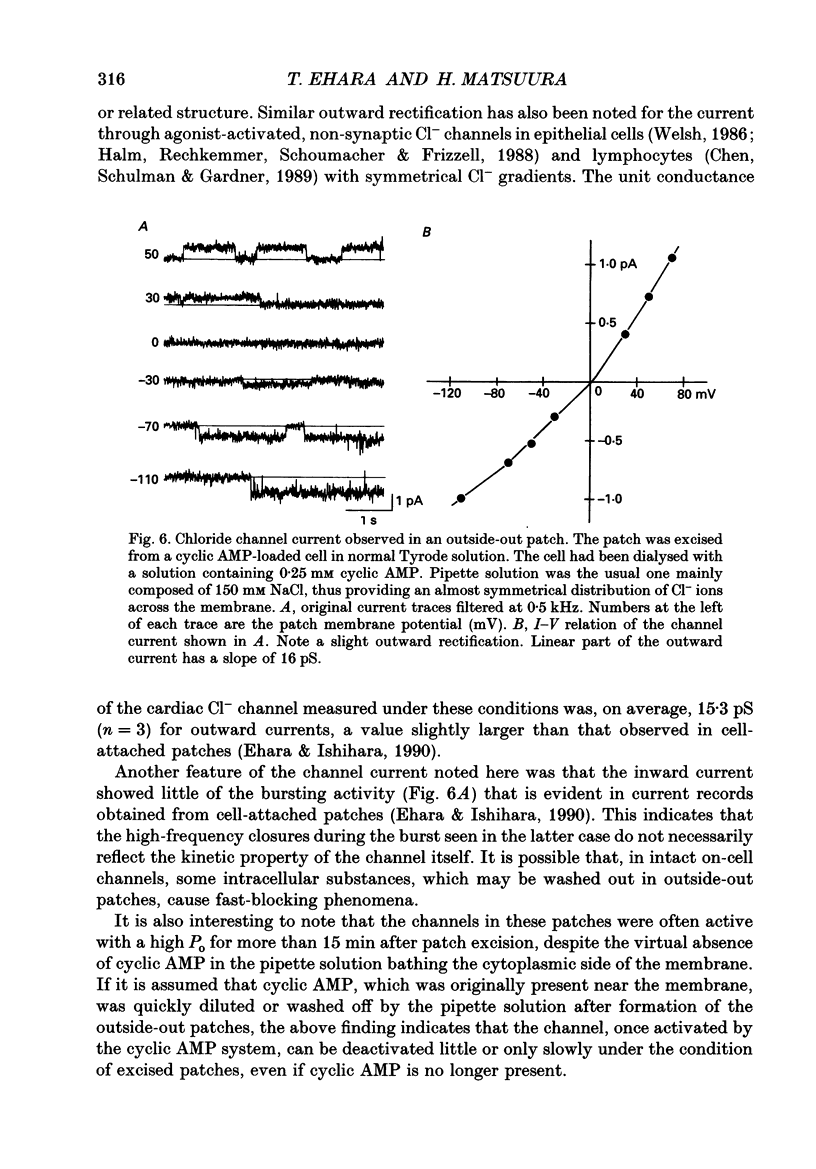
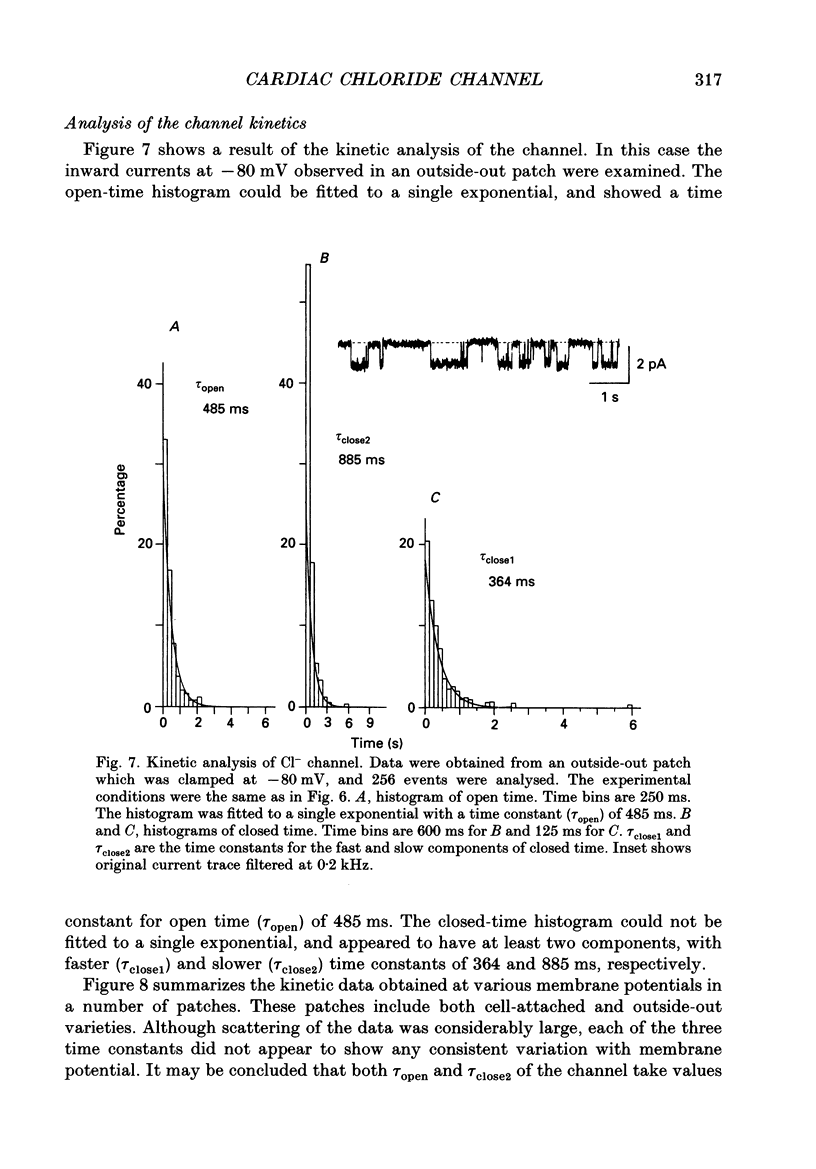
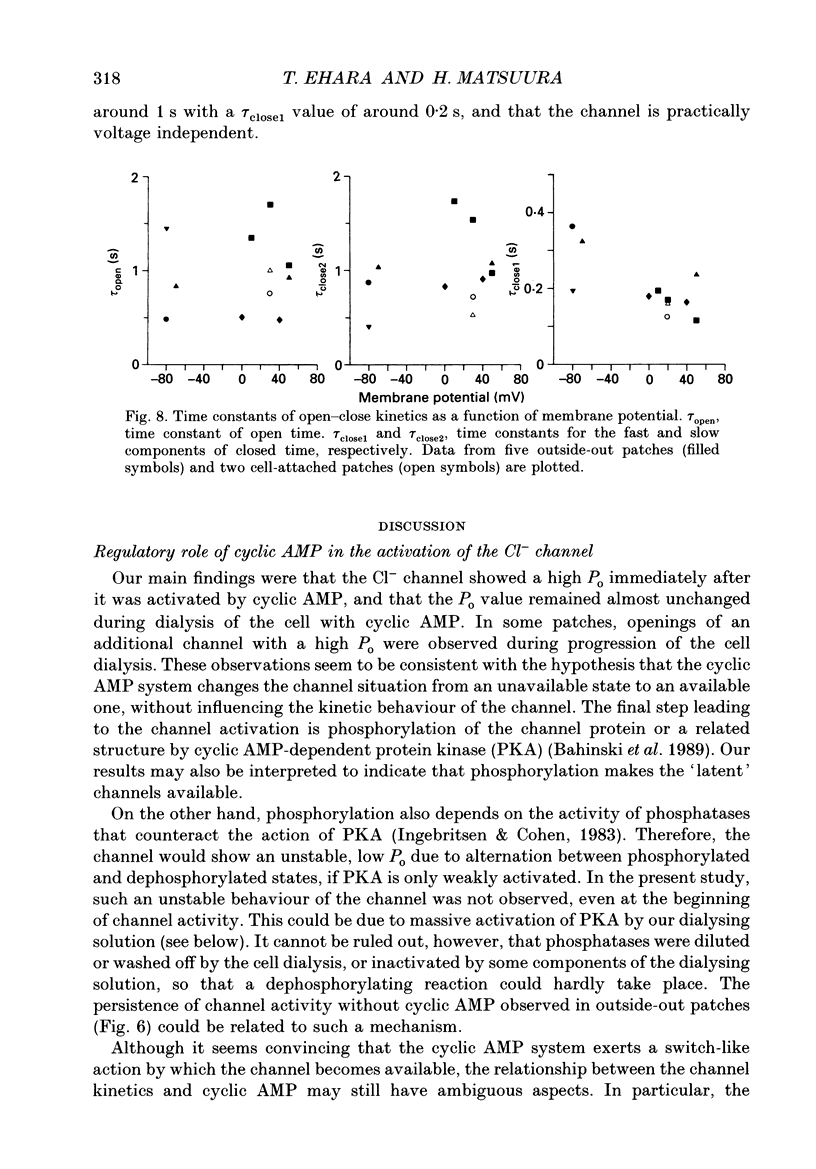
1. Properties of the cyclic AMP-regulated Cl- channel were studied in guinea-pig ventricular myocytes with the patch clamp technique. Cell-attached patch recordings were performed, while the cell was dialysed with a cyclic AMP (0.2-0.5 mM)-containing internal solution through a second patch pipette. The latter pipette was also used to monitor the whole-cell Cl- conductance. 2. The whole cell showed a large Cl- conductance for 10-15 min after the beginning of cell dialysis. The activity of single Cl- channels began to appear in some of the cell-attached patches during this time. 3. The channels showed a high open probability (0.69 +/- 0.14, mean +/- S.D., n = 12) at the time of their appearance, and the open probability did not appreciably increase thereafter, even when the whole-cell Cl- conductance increased further with time. 4. An increase in the number of active channels was observed in some patches with progression of the cell dialysis. In such cases, the newly activated channels also showed a high open probability. 5. The above results are consistent with the hypothesis that the cyclic AMP system makes the 'latent' Cl- channels available without influencing their own kinetic behaviour. The available channels may intrinsically exhibit a high open probability. 6. Chloride channel currents could also be recorded in the outside-out patches excised from the cyclic AMP-loaded cells. The I-V relation of these currents showed outward rectification under the condition of symmetrical Cl- gradients, suggesting that the channel itself or a related structure has the property of rectifying current flow. 7. The channel seemed to have at least one open state and two closed states; the open-time histograms showed one exponential component with the values of time constant scattering around 1 s, while the closed-time histograms showed two exponential components with the values of time constant scattering around 0.2 and 1 s. These time constants showed no clear voltage dependence.
Full text
PDF


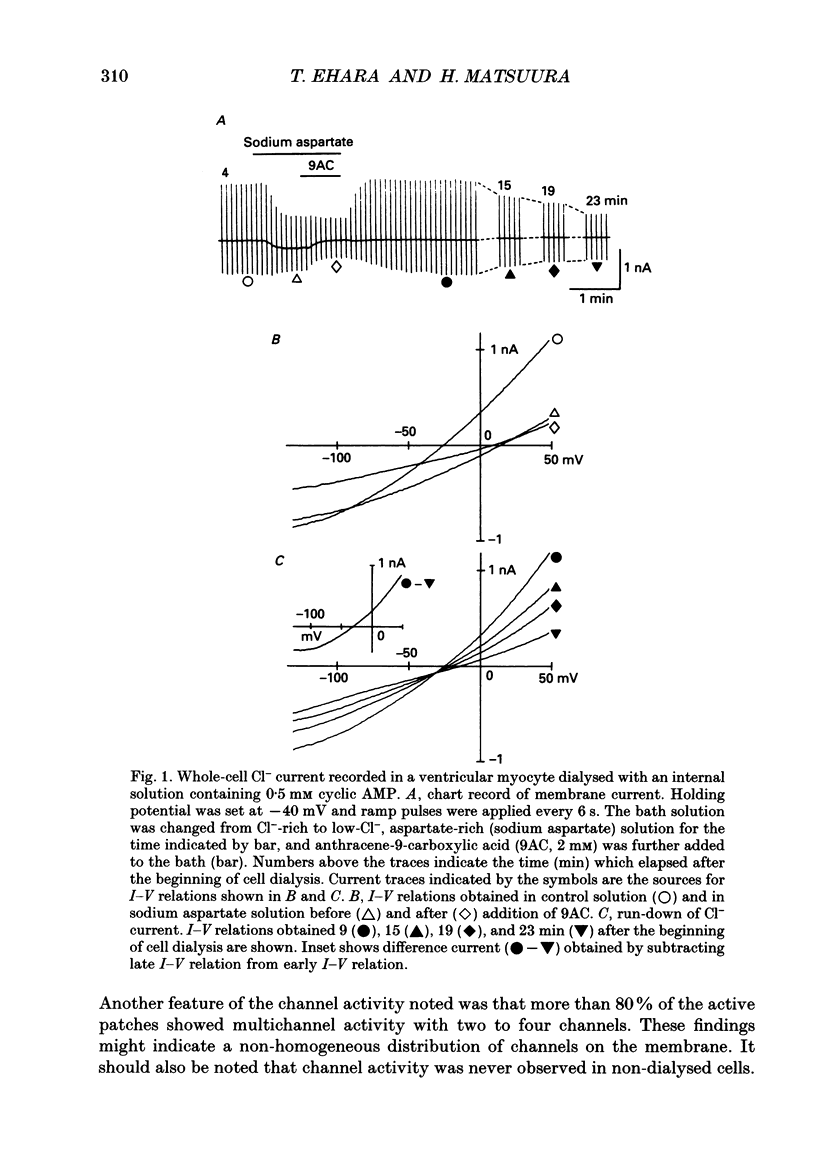
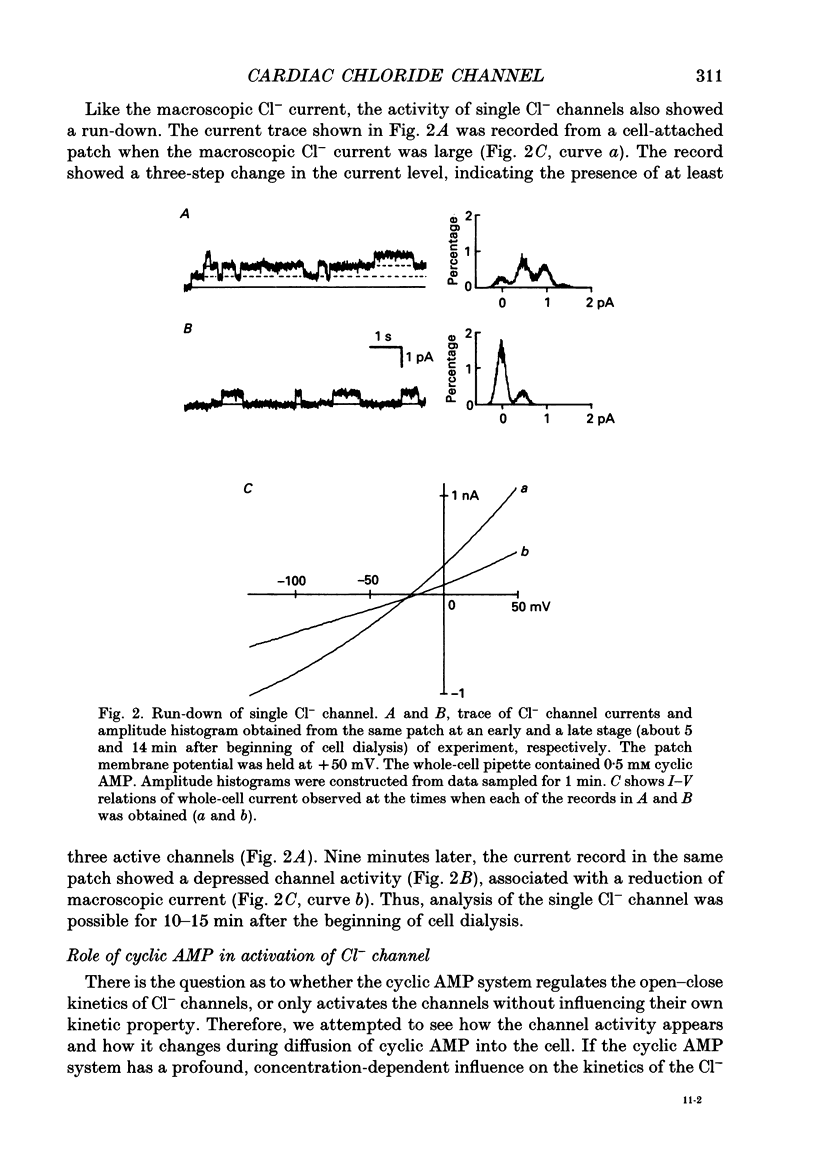
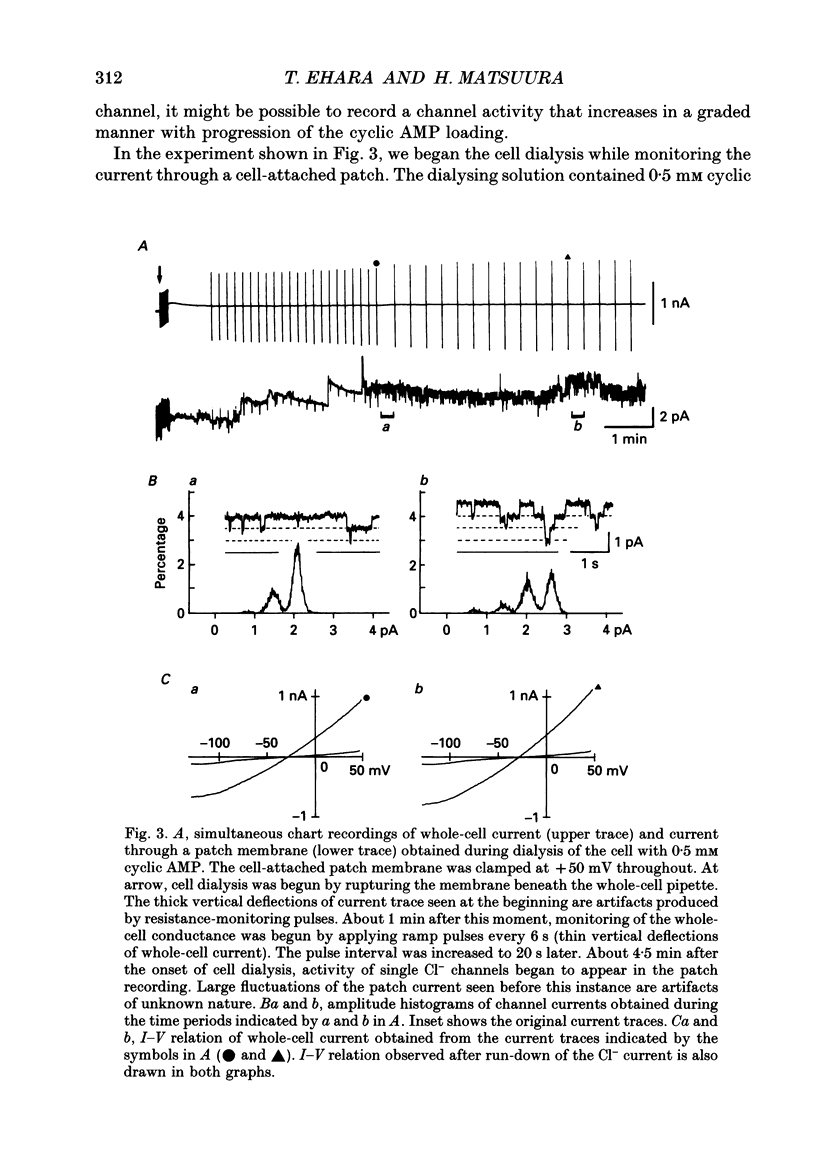

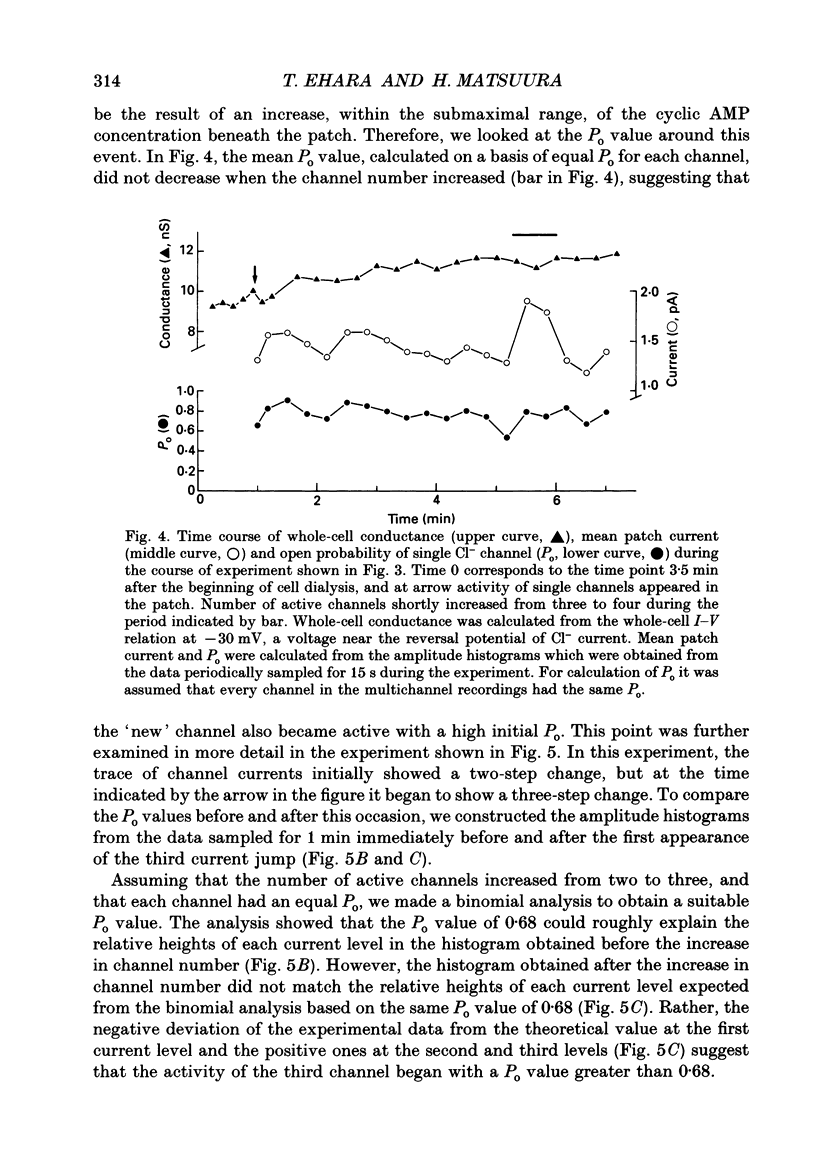


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Noble S. J. Proceedings: Effects of adrenaline on membrane currents underlying pacemaker activity in frog atrial muscle. J Physiol. 1974 Apr;238(1):51P–53P. [PubMed] [Google Scholar]
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Characterization of the acetylcholine-induced potassium current in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:219–237. doi: 10.1113/jphysiol.1986.sp015970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Schulman H., Gardner P. A cAMP-regulated chloride channel in lymphocytes that is affected in cystic fibrosis. Science. 1989 Feb 3;243(4891):657–660. doi: 10.1126/science.2464852. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W. An isoprenaline activated sodium-dependent inward current in ventricular myocytes. Nature. 1987 Aug 13;328(6131):634–637. doi: 10.1038/328634a0. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W., Yamaoka K. On the mechanism of isoprenaline- and forskolin-induced depolarization of single guinea-pig ventricular myocytes. J Physiol. 1988 Jun;400:299–320. doi: 10.1113/jphysiol.1988.sp017121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T., Ishihara K. Anion channels activated by adrenaline in cardiac myocytes. Nature. 1990 Sep 20;347(6290):284–286. doi: 10.1038/347284a0. [DOI] [PubMed] [Google Scholar]
- Ehara T., Matsuoka S., Noma A. Measurement of reversal potential of Na+-Ca2+ exchange current in single guinea-pig ventricular cells. J Physiol. 1989 Mar;410:227–249. doi: 10.1113/jphysiol.1989.sp017530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Shrier A. Interactive effects of isoprenaline, forskolin and acetylcholine on Ca2+ current in frog ventricular myocytes. J Physiol. 1989 Oct;417:213–239. doi: 10.1113/jphysiol.1989.sp017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm D. R., Rechkemmer G. R., Schoumacher R. A., Frizzell R. A. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988 Apr;254(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Effect of forskolin and acetylcholine on calcium current in single isolated cardiac myocytes. Mol Pharmacol. 1987 Nov;32(5):639–645. [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Imoto Y., Ehara T., Matsuura H. Voltage- and time-dependent block of iK1 underlying Ba2+-induced ventricular automaticity. Am J Physiol. 1987 Feb;252(2 Pt 2):H325–H333. doi: 10.1152/ajpheart.1987.252.2.H325. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983 Jul 22;221(4608):331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Ehara T., Noma A. Chloride-sensitive nature of the adrenaline-induced current in guinea-pig cardiac myocytes. J Physiol. 1990 Jun;425:579–598. doi: 10.1113/jphysiol.1990.sp018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H., Ehara T., Imoto Y. An analysis of the delayed outward current in single ventricular cells of the guinea-pig. Pflugers Arch. 1987 Dec;410(6):596–603. doi: 10.1007/BF00581319. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Kokubun S., Prod'hom B. Properties and modulation of cardiac calcium channels. J Exp Biol. 1986 Sep;124:191–201. doi: 10.1242/jeb.124.1.191. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. An apical-membrane chloride channel in human tracheal epithelium. Science. 1986 Jun 27;232(4758):1648–1650. doi: 10.1126/science.2424085. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]


